Abstract
Carriers of a single sickle cell gene mutation generally enjoy normal lifespans without serious health consequences related to their sickle cell status, but under extreme conditions such as severe dehydration and high-intensity physical activity, complications such as exertional rhabdomyolysis, splenic infarction, and papillary necrosis can occur. Recently, the National Collegiate Athletic Association (NCAA) adopted a policy that requires sickle cell solubility testing for all incoming student athletes. However, the American Society of Hematology (ASH) and other physician organizations oppose this policy. What is the basis for this controversy and how have new findings moved the field forward? I discuss herein the epidemiology, genetics, and clinical studies of sickle cell trait; review the implications of current policies regarding sickle cell trait screening and interventions for the student athlete; and examine additional areas where more information is needed.
Introduction
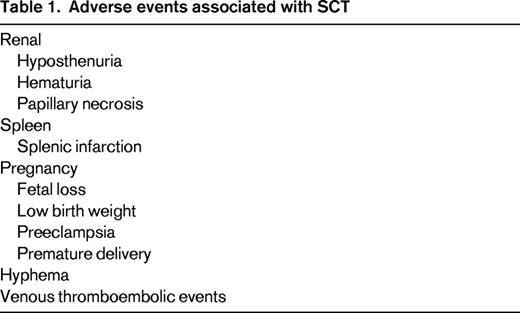
Persons with sickle cell trait (SCT) have inherited a single mutant beta globin allele that, when co-inherited with a second mutant beta globin gene, causes sickle cell disease (SCD). It is estimated that up to 3 million Americans and hundreds of millions of persons worldwide have SCT.1 Most will live normal life spans with no adverse health events related to sickle cell, however, complications have occurred (Table 1). SCT is associated with hematuria, papillary necrosis, and loss of urinary concentrating ability (hyposthenuria). Splenic infarction in SCT occurs under extreme conditions such as high altitudes. Studies of SCT as a risk factor for end-stage renal disease have been conflicting.2,3 SCT has been associated with increased risk of venous thromboembolism,4 but, interestingly, SCT is not a risk for microvascular disease associated with diabetes mellitus.2,5 Complications in SCT were comprehensively reviewed by Key and Derebail for the American Society of Hematology (ASH) Education Program in 2010.6
The National Collegiate Athletic Association (NCAA) estimates that approximately 400 000 student athletes compete each year in sports under its sanction. During a 5-year period from 2004 to 2008 in which athletes logged nearly 2 million participant-years, 273 deaths were reported by the NCAA, with 5 of those deaths occurring in athletes with SCT.7 In April 2010, the NCAA adopted a policy requiring Division I institutions to perform testing for SCT on all incoming student athletes. This requirement arose from the settlement of a lawsuit after the death of a college football player during preseason training. Postmortem investigation into the cause of death revealed that the player had SCT. The policy includes an opt-out provision for students who can provide results from a prior test or for those who are willing to sign a waiver exempting both their university and the NCAA from liability. As of January 2013, similar policies have now been extended to institutions in Divisions II and III. Because the ASH is devoted to the study and treatment of blood disorders, including SCD and SCT, it initiated a process to address the scientific, medical, and ethical issues raised by the NCAA's policy.
ASH conducted a workshop in June 2011 that was aimed at exploring the strength of current scientific evidence in determining whether persons with SCT are at increased risk for exertion-related heat illness or sudden death and whether screening for SCT to participate in athletic programs can be justified by current scientific knowledge. Findings from this workshop greatly informed the ASH policy, which was adopted in 2012. The goals of this presentation will be to describe new relevant scientific data and policy implications of trait testing and describe additional research that is still needed.
Scientific evidence
There are few high-quality (ie, large-scale, well-controlled, hypothesis-driven, prospective) studies of SCT and exertion-related injuries and death. Much of the published information on this topic consists of older data, case series, and studies with very few subjects. SCT does not appear to limit performance among elite athletes in many sports.8,9 A rigorous systematic review of the literature found insufficient evidence to determine that there is an increased risk of exercise-related mortality in persons with SCT. Research examining physiologic mechanisms by which SCT might be linked to exertion-related injury and death or that supports epidemiologic observations of associations with SCT are still evolving.
The risk of SCT among military recruits was demonstrated in the 1980s by Kark et al, who compared deaths in African Americans with SCT with those without SCT during basic training and found a 28-fold increased relative risk of sudden unexplained death that included exertional heat stress, heat stroke, rhabdomyolysis, and causes unknown in recruits with SCT.10 In that study, the manner of death was determined by full autopsies combined with biochemical studies and clinical data. Although the relationship was clear, causality could not be established.
Case-control and cohort studies of physical activity and SCT have examined biomarkers of inflammation, skeletal muscle breakdown, and blood viscosity in trained or sedentary persons with SCT compared with age- and sex-matched subjects without SCT.11,12 No significant differences in physiological responses were detected in exercise studies performed on well-conditioned male students with SCT compared with matched controls. Persons with SCT were capable of adapting to regular physical activity in a manner similar to controls. Oxidative stress responses and nitric oxide metabolism in subjects with SCT not participating in physical activity was shown to be significantly improved without adverse events after an 8-week training program.13 Other studies have shown that moderate endurance training could not induce rhabdomyolysis or renal dysfunction14 and ad lib rehydration after intense periods of physical activity normalized hyperviscosity in persons with SCT.15 Given the sample size and design of these studies, the generalizability of these findings to other athletic activities, conditioning protocols, or to the broader community is not clear.
Deaths of collegiate student athletes in the NCAA are documented in a voluntary memorial list and also through the Parent Heart Watch database, which collects information from media reports, internet searches, and reports from NCAA news, athletic directors, and coroners.7,16 Nonmedical causes (homicide, accidents, suicide) make up more than half of all-cause deaths and 72% of football player deaths. Among football players, the leading medical causes of death are cardiac (45%), heat stroke, and deaths associated with SCT. All 5 of the deaths associated with SCT occurred in black football players during practice and conditioning drills. When exertional death was examined in black Division I football players, the relative risk with SCT was 1:805, 22 times greater in athletes with SCT compared with those without SCT.16
One significant challenge with reconciling published reports relates to event definitions and the lack of uniformity in case ascertainment. Evidence of exercise-related mortality in persons with SCT is made all the more difficult in that sickling observed in autopsy reports can occur as a result of agonal hypoxia alone. Terms such as “intensity syndrome,” “exertional sickling,” “sickle collapse syndrome,” and “sickle trait crisis” are problematic in that they are applied to cases when pathogenesis and causation have not been established and investigation of other contributors may be limited to absent. “Exercise-related adverse health outcome” (ERAHO) and “exercise collapse associated with sickle trait” (ECAST) have been proposed as terminology to reflect the epidemiologic link and the recognition that our current mechanistic understanding requires more research.1,9 Although the spectrum of clinical presentations varies widely, from severe muscle pain to rapidly fatal collapse, the most common settings for ECAST appear to be training and conditioning drills in basic military training (BMT) and in American football. More work is needed to characterize circumstances in which events occur prospectively, including more precise case definitions and comprehensive evaluation of near misses as well as fatalities.
Relationship of exercise, exertional rhabodomyolysis and SCT
SCT is an uncommon but established risk factor for exercise-related rhabdomyolysis and sudden death. Exertional rhabdomyolysis (ER) can cause death in the general population. The absolute risk in the general population and, more specifically, in persons with SCT, is not known. Nearly all reports of deaths in athletes or warfighters with SCT have related to some degree of exertion and have features that resemble ER in many cases.9 Some have not necessarily been in the setting of excess heat and humidity. ER is an acute clinical syndrome caused by the breakdown of striated skeletal muscle due to metabolic derangement or physical injury. Certain forms of high-intensity repetitive exercises and low baseline fitness are common factors in many reports.17 Clinical manifestations include severe pain, muscle tenderness or swelling, dark urine (myoglobinuria), and serum creatine kinase (CK) elevations; however, only 50% of patients have these classic findings at presentation.18 Rhabdomyolysis (exertional and nonexertional) can have multisystem consequences or complications such as acute kidney injury, disseminated intravascular coagulopathy, hyperkalemia, and cardiac dysrhythmias. Successful treatment includes early detection addressing the underlying cause, measures to prevent renal failure, and correction of metabolic derangements. Factors associated with ER are listed in Table 2.
US Armed Forces researchers have performed population-based and more targeted clinical, biochemical, and genetic cohort studies of ER. Studies of all-cause rhabdomyolysis and ER among recruits in BMT have identified some key differences. The incidence of ER was 22.2 cases per 100 000 trainees per year. Trainees with ER tend to be significantly younger (mean age 23.3 vs 53.8 years), are more likely to be male (88.9 vs 74%), and experience less acute renal failure (19.1 vs 34.2%) compared with those with rhabdomyolysis from other causes.19 Military trainees with ER were not significantly different from trainees without ER in terms of age, sex, body mass index, or summer season for BMT. Recurrences were infrequent. The incidence of ER is higher in males, persons of African descent, recruits in BMT, members of the Army and Marine Corps, and in combat-specific occupations.20 Annual rates of ER in the military have increased by 30% from 2008 to 2012, prompting reexamination of preventive and management strategies.
Serum CK will increase in all humans after strenuous exercise.17 Baseline levels of CK are higher in men than in women and in African Americans compared with whites. Genetic susceptibility to ER has focused on candidate genes that are involved in CK expression, muscle metabolism, heat injury, and renal function. Inherited mutations that result in deficiencies of glycogen myophorylase (McArdle disease), carnitine palmitoyl transferase, and myoadenylate deaminase are associated with myalgias, reduced exercise tolerance, and rhabdomyolysis.17 Analysis of ER cases compared with healthy persons undergoing a laboratory-controlled exercise challenge identified specific genetic polymorphisms in CKKM, ACTN3, and MYLK2 that were associated with ER.21 In that study, 10.6% of the ER cases were SCT positive. Several mutations in the ryanodine receptor type 1 (RYR1) gene, which are considered causative in malignant hyperthermia, have also been identified in African-American men with ER.17,22 These studies underscore the potential for alternate hypotheses in which SCT is either a surrogate marker or its pathogenetic role is in combination with other variables.
Risk mitigation: the US military approach
Although the US Armed Forces recognizes the increased risk of exertional heat-related injuries (EHI) in recruits with SCT compared with those without EHI, selected branches of the US military have used a universal strategy for prevention of exertion-related heat illnesses during basic training and have almost eliminated deaths in all trainees. More recent unpublished data from the Armed Forces examined whether measures to reduce EHI could affect mortality irrespective of SCT status.23 This study showed that preventive measures used by military branches to mitigate risk for all soldiers, such as heat acclimatization, monitoring work-rest cycles, guidelines for nutrition and hydration, and maintaining staff preparedness for early and rapid detection and treatment of heat illnesses, could significantly reduce EHI in all persons regardless of hemoglobin status. Repeated, high-intensity timed drills with limited recovery time are avoided in the first 1 to 2 weeks of BMT.9 Given past associations of physical readiness testing (PRT) with death in persons with SCT, a minimum 48-hour time interval is required between strenuous field training and PRT. Using these universal guidelines, the death rate has been drastically lowered and the previously reported increased risks due to SCT are no longer apparent. A memorandum from the Secretary of Defense in place since 1996 states that SCT testing is not required for military accession, but testing may be performed after accession for specialized occupations. The general military medical screening guidelines have been deemed sufficient to identify persons with SCD. Similarly, the Brazilian government has promulgated guidelines to avoid heat-related injuries and no longer sees an increased risk for athletes or soldiers with SCT.
Currently, there are no evidence-based guidelines for managing ECAST. Recommendations for early detection and treatment of ECAST that could be applied to both the warfighter and athlete have been proposed and are under development.9,17 One critical step is the establishment of a communication plan for emergency services with a receiving facility that has the capacity to evaluate and effectively treat rapidly progressive metabolic derangements and organ dysfunction. It is not clear that any of the recommendations for medical management are specific to SCT. At present, there is also no consensus on the timing and circumstances of return to play/return to duty after an episode of ER or ECAST.24
Public health considerations
Universal newborn screening for SCD in the United States is performed as a public health imperative because early detection of SCD and intervention reduces morbidity and mortality in young children. This screening strategy detects persons with SCT. However, many young persons in the United States who were screened for SCD as newborns may not know if they have SCT. Principles of public health ethics include an obligation that public health policies should minimize the burden imposed on persons to effectively meet public health goals. The scale and scope of screening undertaken by the NCAA does not adhere to this standard. Given the current state of the scientific evidence, it is not clear that the approach implemented by the NCAA is the least burdensome policy option available that can effectively meet the goal of protecting athletes with SCT from exertion-related illness. Calls for universal precautions applied to all athletes, regardless of their trait status, embodies a profile of potential benefits that may make athletic participation safer.
Legal and ethical considerations
The legal, ethical, and societal implications of SCT screening were considered in recent workshops.1,8 Establishment of a policy without supporting medical evidence may place the student athlete and the larger community of persons with SCT at risk of ethical harms, such as loss of privacy, stigmatization, and discrimination. The expressed expectation that NCAA student athletes should set their own pace during workouts and take extended periods of rest between activities if needed may easily distinguish athletes with SCT from their teammates if their teammates are not allowed these same accommodations. This could conceivably affect the perceptions of coaches, athletic trainers, or teammates about the person's athletic ability or commitment. Athletes with SCT could potentially lose their scholarships or see reduced playing time due to the perceived need to “protect the athlete with trait” and/or to “protect institutions from liability” should a health complication develop.
Policy positions of ASH and other organizations
ASH.
ASH does not support the NCAA policy because it is not based on scientific evidence that justifies mass screening for SCT in order to participate in college athletics. The recommended sickle solubility test lacks sufficient specificity and may give misleading results. The ASH policy maintains that the current NCAA framework for sickle cell screening does not comply with best practices for testing persons for inherited conditions with appropriate safeguards for genetic information and assurances about counseling and education. The ASH policy asserts that implementation of universal guidelines to reduce exertion-related injuries can be an effective approach for all athletes irrespective of their sickle cell status. ASH is concerned about potential inadvertent harm to the student athlete and the larger community, including stigmatization and discrimination. The wide-ranging media reports on sickle cell trait have lead to generalizations about testing and concerns about physical activity in otherwise healthy children and adults with sickle cell trait. Finally, the ASH policy strongly supports biomedical and population-based research that can facilitate more rational clinical interventions and policy development. Other organizations have addressed concerns about SCT and athletic participation (Table 3).
SCDAA
In May 2011, The Medical and Research Advisory Committee (MARAC) of the Sickle Cell Disease Association of America (SCDAA) released sickle cell carrier screening recommendations for any athletic programs that choose to follow the NCAA ruling. They concluded, given the lack of scientific evidence that substantiates a significant correlation between SCT in athletes and training-related sudden death, that SCDAA does not support screening of athletes for SCT as a means to reduce heat-related illness or death in athletes who are carriers. SCDAA also supports the implementation of universal, safe training guidelines for all athletes; to rigorously educate and improve the capacity of athletic coaches and trainers to recognize the signs and symptoms of heat-related illness; and to provide medical care to athletes who become ill or injured under their supervision.
SACHDNC
The US Department of Health and Human Services (DHHS) Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) developed a report in 2010 on screening of US college athletes for their SCD carrier status.25 Although persons should understand the medical and genetic consequences of SCT, the committee recommended that genetic testing should not be a prerequisite for participation in sports unless deemed medically necessary, and that evaluation should include counseling with assurances about the privacy of genetic information. The committee also recommended that all athletes be given education on safe practices for the prevention of exercise- and heat-related illnesses. These recommendations were formally endorsed by DHHS Secretary Kathleen Sebelius in June 2011.
ACSM
The American College of Sports Medicine (ACSM), which includes many subspecialists who serve as collegiate team physicians, does not have a formal position on SCT screening as a prerequisite for athletic participation; however, they believe that if screening occurs, it should be voluntary and persons should be offered counseling to include family planning. Unwarranted limitations of activities should not be placed on the athlete with SCT. Student-athletes are encouraged to avoid dehydration, gradually acclimate to heat and new exercise routines, and to refrain from exercise during acute febrile illnesses.
Brazilian guidlines
The sickle gene frequency in Brazil is similar to that in the United States, prompting representatives of the Brazilian Armed Forces, sports organizations, and sickle cell experts and other stakeholders to consider implementation of a wide-reaching policy in 2007. A consensus white paper was generated in which they concluded that there is no need to perform screening for hemoglobinopathies of persons in professional or amateur sports, nor is screening necessary to serve in the Army. They emphasized the need to raise awareness among the general population that, as long as basic hydration and rest recommendations were followed, persons with SCT can safely participate in physical activities.
AAP
The American Academy of Pediatrics (AAP) has not developed a specific position on SCT and athletic participation, but they recently released a policy statement on ethical issues of genetic testing that includes general language regarding carrier screening.26,27 In collaboration with the American College of Medical Genetics and Genomics (ACMG), AAP does not support screening solely to determine carrier status unless the results have clear health benefits. Testing or screening should be driven by the best interest of the child. They advise against school-based screening because the school environment is not conducive to voluntary participation, where thoughtful consent, confidentiality, and appropriate counseling can be ensured.
Future directions: a way forward
The National Heart, Lung, and Blood Institute of the National Institutes of Health and the Centers for Disease Control and Prevention have convened consensus conferences recently to frame a SCT research agenda.1,8 The Consortium for Health and Military Performance also organized a roundtable with representatives from the US Department of Defense, ACSM, NCAA, and content experts from the ASH to examine strategies to mitigate risk associated with SCT.9 The best available evidence suggests that under extreme conditions such as vigorous physical activity with severe dehydration, SCT increases the relative risk of heat-related illness and death; however, the overall absolute risk is believed to be low. It remains unclear why some persons participating in competitive sports or in the military develop ER whereas others under similar conditions of physical exertion do not. Plausible mechanisms have been proposed, but they are as yet untested6,11,28 and focused research is needed to explore genetic, biologic, and environmental factors. Data from new sources, including additional information from efforts by the military to mitigate heat-related risk; consistent comprehensive testing of athletes who experience severe myalgias or frank ER; and qualitative research on the impact of the NCAA mandate on student-athletes are likely to make important contributions to more rational, evidence-based policy. Based on current evidence, universal precautions that mitigate risk of exertion-related injuries and deaths and have no potential to harm may be the more prudent path.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Novartis, Glaxo Smith Kline, Eli Lilly, Amgen, and bluebird bio; has consulted for Novartis and ApoPharma; and has received honoraria from ApoPharma. Off-label drug use: None disclosed.
Correspondence
Alexis A. Thompson, MD, MPH, Lurie Children's Hospital Chicago, 225 E Chicago Avenue, Box 30, Chicago, IL 60611; Phone: 312-227-4834; Fax: 312-227-9756; e-mail: a-thompson@northwestern.edu.