Abstract
Mantle cell lymphoma (MCL) is a unique subtype of non-Hodgkin lymphoma that is both biologically and clinically heterogeneous. A variety of biomarkers, the achievement of minimal residual disease negativity after initial therapy, and the MCL International Prognostic Index (MIPI) are associated with patient outcome, although none has as yet been used for routine treatment stratification. Given the lack of widely accepted and standardized treatment approaches, clinical trial enrollment should always be considered for the initial therapy of MCL. Outside of the trial setting, younger and transplantation-eligible patients with newly diagnosed MCL who require treatment should first be considered for a rituximab + a high-dose cytarabine–containing regimen, followed by autologous stem cell transplantation consolidation in first remission. Symptomatic elderly and nontransplantation-eligible individuals typically receive rituximab + bendamustine, or R-CHOP (rituximab + cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone/prednisolone) followed by maintenance rituximab, the latter a treatment plan that has demonstrated extended response duration and survival. Promising early results for consolidation approaches with proteasome inhibitors and immunomodulatory drugs are now being tested in randomized clinical trials. The availability of highly active BCR signaling pathway inhibitors and cell death pathway modulation via BH3 mimetics, among other novel agents, promise to rapidly expand treatment options, change existing treatment paradigms, and further improve outcomes for MCL patients.
Introduction
Remarkable progress has been made in the biologic and clinical understanding of mantle cell lymphoma (MCL) since it was first defined as a unique pathologic and clinical entity 20 years ago. Nonetheless, MCL remains a considerable challenge due to its marked clinical heterogeneity and the lack of generally accepted standards of care. For most patients, it remains an incurable disease, albeit with much improved response durations and overall survival (OS) in recent years. This review summarizes recent phase 3 data in frontline therapy, discusses the current role for consolidative hematopoietic stem cell transplantation (SCT), identifies emerging regimens and novel agents that may lead to effective alternatives to SCT and describes ongoing studies of prognostic markers for risk-adapted treatment approaches. Although a comprehensive discussion of these topics is beyond the scope of this review, several excellent reviews have been published recently.1-4
Biological and clinical spectrum of MCL
MCL comprises 5% to 6% of new non-Hodgkin lymphoma diagnoses in the United States, ∼ 5000 new cases annually. Patients are typically > 60 years of age, with a male predominance and advanced-stage disease at diagnosis. Involvement of the BM, peripheral blood, and extranodal sites, especially the gastrointestinal tract and soft tissue sites, is the norm.
MCL is characterized in virtually all cases by the chromosomal translocation t(11;14)(q13;q32) and nuclear cyclin D1 expression, rendering pathologic diagnosis relatively straightforward in most cases. Heterogeneity exists at both the cellular and molecular levels, which in turn have prognostic implications. Patients with the blastoid cell type and diffuse morphology typically have inferior outcomes; conversely, a nodular or mantle zone growth pattern is often reflected by slower-paced disease. Approximately 20% of MCL patients are clinically “indolent” and may be managed by watchful waiting.5 These patients often present with a chronic lymphocytic leukemia (CLL)–like picture of leukemic phase and splenomegaly with absent or low-tumor-burden adenopathy. In these cases, the inclusion of t(11;14) analysis in a CLL FISH panel helps to prevent misdiagnosis. Biomarkers of indolent MCL include mutated immunoglobulin heavy chain (IGH) variable genes, lack of expression of the nuclear protein Sox11, and relatively low expression of the proliferation marker Ki-67. The more frequently encountered “aggressive” MCL presents with high-tumor-burden nodal disease, high Ki-67 score, mutated p53 or Notch 1, an altered microRNA signature, and a clinical course characterized by short response duration and decreased survival. A high Ki-67 score has been shown to have a strong correlation with poorer outcome in several studies, although the lack of standardized methods for Ki-67 staining and interpretation, as well as a lack of established expression cutoff levels, currently limit use of the score for individual patient management.
The challenge of MCL heterogeneity and treatment selection has also been addressed by clinical prognostic scoring systems. The most robust of these is the MCL International Prognostic Index (MIPI), which uses patient age, performance status, serum lactate dehydrogenase, and total WBC count to stratify patients into low-, intermediate-, and high-risk groups.6 The addition of Ki-67 scoring provides a biologic MIPI that further improves prognostic stratification. The MIPI has been validated in several large clinical trial cohorts, including those using SCT consolidation therapy, and should be calculated for all newly diagnosed patients. However, MIPI-based treatment recommendations remain to be established by ongoing prospective trials.
It is important to recognize that most patients with MCL require treatment upon presentation due to symptomatic disease from B-symptoms, bulky lymphadenopathy or splenomegaly, extranodal involvement, or cytopenias. The following sections summarize current therapeutic approaches for both SCT-eligible and SCT-ineligible patients, as well as current efforts to improve progression-free survival (PFS) and OS.
Frontline treatment of MCL
To date, standard immunochemotherapy regimens have proven noncurative for most MCL patients. Although high overall response rates (ORR) can be achieved with a variety of rituximab + chemotherapy regimens, the response durations have generally been only 18 to 24 months, with OS in the range of 4 to 7 years. Several approaches have been pursued in an effort to improve PFS and OS, including the use of high-dose cytarabine-containing regimens, consolidative autologous SCT (ASCT) in first complete remission (CR1), novel agents alone or in combination with R-chemotherapy regimens, and maintenance rituximab. Patients who are medically fit, 65 to 70 years of age or younger, and who are eligible for dose-intensive therapy are the focus of the following section.
High-dose cytarabine and ASCT
Several lines of evidence support an important role for high-dose cytarabine (HiDAC) in prolonging initial treatment response, if not OS. A single-institution study of R-HyperCVAD (rituximab + fractionated cyclophosphamide + vincristine, doxorubicin, and dexamethasone) alternating with R + high-dose methotrexate and HiDAC showed a 97% ORR with 87% CRs.7 With a median follow-up of 8 years, the median time to treatment failure was 4.6 years (5.9 years for patients < 65). However, multicenter trials have found lower responses and high toxicity rates, with more than one-third of patients unable to complete the planned therapy.8,9 Of concern, the current U.S. Intergroup trial (SWOG 1106) of R-HyperCVAD/MTX-AraC versus R-bendamustine followed by ASCT was closed recently due to an impaired ability to collect sufficient stem cells from patients in the R-HyperCVAD arm. Given the toxicity of this regimen and the need for inpatient chemotherapy administration (and frequently for management of cytopenic or other treatment-associated complications), alternative HiDAC-containing regimens should be used for MCL therapy.
R-DHAP (R + dexamethasone, HiDAC, cisplatinum) showed a CR/unconfirmed CR rate of 76% in 199 untreated MCL patients after 4 cycles of therapy, with most then proceeding to ASCT.10 The Nordic group also reported high pre-ASCT responses when HiDAC and R were added to their earlier CHOP-based regimen (cyclophosphamide, doxorubicin, vincristine, prednisone).11 The French/Belgian Groupe d'Etude des Lymphomes de l'Adulte (GELA) conducted a phase 2 trial of 60 previously untreated patients < 66 years of age with advanced MCL using R-CHOP × 3 cycles followed by 3 cycles of R-DHAP. A total of 93% of patients responded to R-CHOP, but only 12% achieved CR. This increased to 57% after R-DHAP.12 Seven patients did not complete planned therapy, 3 due to disease progression and 4 due to toxicity. Forty-nine patients underwent ASCT, with only 1 patient failing stem cell collection. With a median follow-up of 67 months, the 5-year OS is 75%. No myelodysplastic syndrome or acute myeloid leukemia events were reported, although there was a surprisingly high rate of second cancers (11 patients, 5 with renal cell carcinoma).
In the largest phase 3 study to date, the European MCL Network compared induction therapy with R-CHOP × 6 cycles versus a regimen of alternating R-CHOP and R-DHAP (3 cycles each); each study arm was followed by ASCT consolidation for responding patients (the “MCL Younger Trial”).13 The pre-ASCT conditioning regimens differed: R-CHOP–treated patients were mobilized with DexaBEAM followed by a conditioning regimen of cyclophosphamide plus total-body irradiation, whereas the R-CHOP/R-DHAP patients were collected after the final cycle of R-DHAP followed by conditioning with HiDAC, melphalan, and a lower-dose total body irradiation regimen. A total of 497 previously untreated patients < 66 years of age were randomized, with 455 evaluable. The pre-ASCT ORRs were 90% to 95%, with higher CR in the R-CHOP/R-DHAP arm (36% vs 25%, P = .0003). A total of 72% of patients in each arm underwent ASCT, with final CR rates of 63% and 61% (P = NS). At a median follow-up of 51 months, the remission duration was significantly improved in the R-DHAP–containing arm (84 months vs 49 months; P = .0001) with a benefit in OS (not reached vs 82 months, P = .045). Hematologic and renal toxicity were higher during the induction phase with the alternating regimen compared with R-CHOP. The benefit of dose-intensive therapy and ASCT was observed across the clinical spectrum of MCL, although it was greater in those with low- or intermediate-risk MIPI than in those with high-risk scores. The investigators concluded that HiDAC is a key component of initial therapy for eligible MCL patients and that ASCT in CR1 is a current standard of care, a position supported by National Comprehensive Cancer Network 2013 recommendations and by the European Society for Medical Oncology 2013 Consensus Conference.
Should molecular remission be a goal of therapy?
The achievement of minimal residual disease (MRD) and molecular remission are well-established in CML and acute lymphocytic leukemia as important determinants of patient outcome and as a therapeutic guide. To date, MRD testing is not fully established in MCL, lacking both standardized methodology and availability outside of clinical trials. However, achievement of molecular remission was an independent and very strong predictor of clinical outcome in the MCL Younger Trial described above and in the European MCL Network Elderly Trial of frontline immunochemotherapy (see “Treatment of MCL in non-SCT-eligible patients” below).
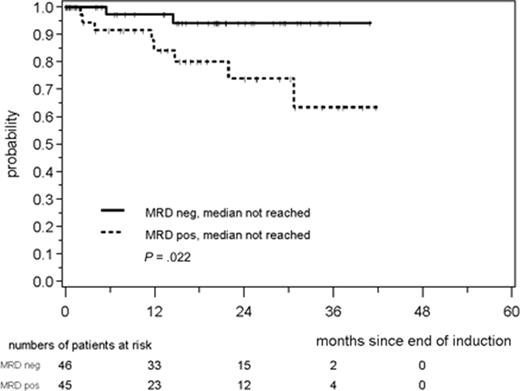
In the MCL Younger Trial, peripheral blood and BM samples were obtained at baseline and at various time points during induction therapy, pre- and post-ASCT, and during follow-up. MRD analysis was performed by RQ-PCR for clonal IGH rearrangements and for the t(11;14) translocation (BCL1-IGH) and compared with standard clinical response criteria and patient outcome.14 A total of 90% of patients had a detectable MRD marker at baseline. After induction therapy, BM MRD negativity was significantly greater in the R-CHOP/R-DHAP–treated patients (68%) versus those treated with R-CHOP (24%). ASCT improved MRD BM negativity to 65% of patients for R-CHOP and 88% for R-CHOP/R-DHAP, verifying a benefit for ASCT consolidation in deepening remission. The achievement of MRD negativity after induction therapy improved remission duration at 2 years compared with those who remained MRD positive (89% vs 74%, P = .0017; Figure 1). Sustained molecular remission in the peripheral blood during the first year after ASCT was correlated with improved time to treatment failure and a 2-year PFS of > 90%. MRD status was the strongest independent predictor of outcome in this trial and was superior to baseline MIPI status, treatment type, or achievement of CR by standard imaging. Sequential MRD analysis during and after therapy thus holds strong promise for predicting outcome and for modifying patient management, such as the use of rituximab “preemptive therapy” for molecular relapse to reinduce remission.15 Standardization of sampling time points, sample collection and processing, and molecular methods will be necessary. In addition, prospective testing to validate treatment intervention based upon MRD results will be essential.
Response duration according to MRD status in peripheral blood and/or BM after induction immunochemotherapy in the European MCL Younger Trial. Used with permission from Pott et al.10
Response duration according to MRD status in peripheral blood and/or BM after induction immunochemotherapy in the European MCL Younger Trial. Used with permission from Pott et al.10
What is the role of allogeneic SCT?
Allogeneic SCT is recognized as the only proven approach to cure in MCL. However, only a minority of patients are eligible due to the attendant short- and long-term risks of the procedure and the typically older age and comorbid illness of many MCL patients. It is not recommended as part of frontline therapy or consolidation outside of a clinical trial. Retrospective single- and multi-institutional reviews have shown durable PFS of 14% to 46% and OS of 37% to 53%, with evidence of a plateau in the survival curve suggesting cure.16-18 One-year nonrelapse mortality was 18% to 22%. A beneficial effect of donor lymphocyte infusion was documented in some relapsing patients, consistent with a GVL effect. Long-term responders were observed among patients failing prior ASCT and multiple lines of prior therapy.
An analysis from the Center for International Blood and Marrow Transplant Research database identified 202 patients with treatment-refractory MCL who underwent myeloablative (n = 74) or reduced-intensity conditioning/nonmyeloablative transplantations (n = 128).19 There were no significant differences between the conditioning regimens at 3 years, with nonrelapse mortality at 43% to 47%, relapse or progression in 32% to 33% of patients, PFS 20% to 25%, and OS 25% to 30%. Higher mortality was observed with the use of BM as the stem cell source or with T-cell–depleted grafts.
The option of allogeneic SCT should always be addressed in a younger, otherwise healthy individual at the time of first relapse or disease progression, including those with prior ASCT, as the only established curative approach. Chemosensitive patients appear to have better outcomes. The use of one or more of the novel therapeutic agents described below as a bridge to allogeneic SCT also appears promising and may lower the non-treatment-related mortality associated with traditional cytotoxic salvage regimens.
Treatment of MCL in non-SCT-eligible patients
The majority of newly diagnosed MCL patients are not candidates for dose-intensive therapy and ASCT due to age and comorbidities. Although no clear standard of care exists for those patients requiring therapy, the accepted approach until recently has been immunochemotherapy induction followed by observation and sequential treatment of relapsing disease. The proteasome inhibitor bortezomib and the immunomodulatory agent lenalidomide are approved for relapsed disease in the United States, whereas the mammalian target of rapamycin (mTOR) inhibitor temsirolimus is approved in the European Union. Several clinical trials have explored the integration of these agents concomitantly with R-CHOP or R-bendamustine and as consolidative therapy, but these approaches remain investigational.
R-CHOP has been the most widely used regimen in MCL, but median response durations have been on the order of 18 to 24 months only. The high activity of bendamustine in relapsed MCL led to a German multicenter phase 3 noninferiority trial comparing R-CHOP with R-B in patients with MCL or indolent B-cell lymphoma.20 MCL patients were > 65 years of age or ineligible for ASCT. Treatment consisted of standard R-CHOP-21 versus bendamustine 90 mg/m2 on days 1 and 2 of each 28-day cycle, with standard-dose R on day 1. A total of 46 MCL patients were randomized to R-B and 48 to R-CHOP, with a median PFS of 35.4 months versus 22.1 months, respectively (P = .0044). Lower toxicity was observed for R-B, including significantly less grade 3-4 neutropenia despite less frequent use of G-CSF, as well as fewer infectious complications, less peripheral neuropathy, and no alopecia. R-B is now accepted as a frontline regimen for MCL and is the therapeutic backbone in the current Eastern Cooperative Oncology Group (ECOG)/Intergroup trial for MCL patients > 60 years of age.
The multicenter phase 3 MCL Elderly Trial compared R-CHOP-21 × 8 cycles with R-fludarabine plus cyclophosphamide (R-FC) every 28 days × 6 cycles as induction therapy for non-SCT-eligible patients age 60 or older with previously untreated MCL. Responding patients underwent a second randomization to thrice-weekly versus maintenance R given once every 2 months.21 Maintenance was continued until disease progression or toxicity. A total of 532 randomized patients were included in the intent-to-treat analysis and 485 in the primary analysis. The ORR after induction therapy was 86% for R-CHOP (n = 246) and 78% for R-FC (n = 239), with CR rates of 34% and 40%, respectively (P = .10). Four-year OS was significantly poorer with R-FC (47%) versus R-CHOP (62%; P = .005). More patients progressed during therapy with R-FC (14% vs 5%) and more patients died of lymphoma, infection, or second primary malignancy after that regimen. A significant benefit was observed for both PFS and OS for maintenance R after R-CHOP (but not R-FC) compared with maintenance IFN-α. The investigators concluded that R-CHOP followed by maintenance R is an effective regimen for older, non-SCT-eligible patients.
Maintenance therapy: evolving options
R-CHOP followed by maintenance R in the MCL Elderly study showed that the median remission duration was not reached at 36 months median follow-up compared with 23 months for patients receiving IFN-α maintenance, the latter very similar to prior published reports of remission duration with R-CHOP alone. At a median follow-up for OS of 42 months, there was a significant benefit for R maintenance, with median OS not reached, versus 64 months with IFN-α maintenance (P = .005). Kenkre et al22 used modified R-HyperCVAD induction therapy (without methotrexate or cytarabine) in 22 non-SCT-eligible patients. Induction therapy was followed in those patients achieving CR or partial remission (PR) by maintenance R administered weekly × 4 doses every 6 months for 2 years. With a median follow-up of 62 months, the median PFS was 37 months and the median OS 70 months, with no late toxicities. The response durations in these trials thus compare favorably with those after SCT consolidation, although prospective comparison will be necessary to discern the relative benefit and safety of these 2 postinduction approaches.
The immunomodulatory agent lenalidomide has single-agent activity in relapsed or refractory MCL, including patients treated with prior bortezomib, and has been approved for this indication by the Food and Drug Administration (FDA).23 Recent preclinical and clinical data in CLL and indolent lymphoma also have shown synergy for lenalidomide in combination with R, based at least in part on enhancement of immune synapse formation of effector T cells with R-labeled tumor cells.24,25 The current Intergroup trial (ECOG 1411) for MCL patients 60 years or older will further evaluate the maintenance question. This study uses R-bendamustine induction with or without bortezomib, followed by maintenance therapy with R versus R-lenalidomide (the so-called R2 regimen). PET imaging and MRD testing are included as correlative response evaluations in this trial.
For the present, clinical trials should always be considered in the initial treatment of MCL. Outside of the trial setting, younger and SCT-eligible patients with newly diagnosed MCL who require treatment should be first considered for a Hi-DAC-containing regimen (in my opinion, preferably R-CHOP alternating with R-DHAP rather than R-HyperCVAD/R-MTX-HiDAC due to short- and longer-term toxicities and impaired stem cell collection), followed by ASCT in first remission. Older and non-SCT-eligible individuals should receive R-chemo, preferably with R-bendamustine, or with R-CHOP followed by maintenance R. The use of maintenance R after R-bendamustine awaits confirmation of efficacy and safety by ongoing phase 3 clinical trials.
Novel targeted therapeutics: changing the MCL treatment paradigm
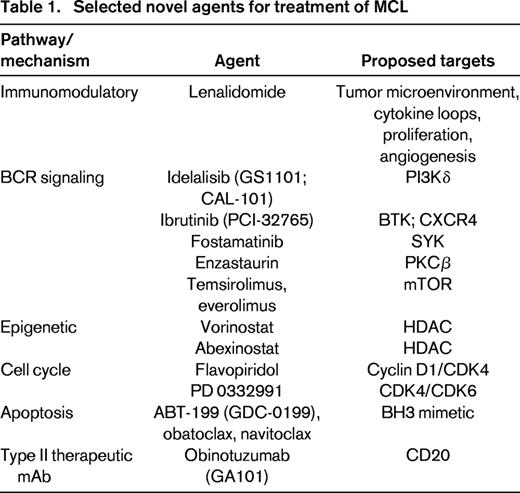
Although MCL responds well to initial therapy, most patients relapse within 1 to 5 years after induction therapy. Second-line regimens can show high therapeutic activity, although the durability of these responses is often short lived. There has been a recent emergence of many novel agents with diverse mechanisms of action, some targeted to the BCR signaling pathway, dysregulated cell cycle elements, or to proliferation or apoptosis pathways. Several of these agents have shown remarkable clinical activity and are being incorporated into frontline regimens either as a component of combination therapy or via maintenance or consolidation strategies (Table 1).
Immunomodulatory drugs
Lenalidomide has activity in relapsed or refractory MCL and is now approved by the FDA for this indication. Mechanisms include direct antiproliferative activity, down-regulation of tumor cell/stromal cell interactions with disruption of essential microenvironmental cytokine loops, and immunomodulatory and antiangiogenic effects. Among heavily pretreated MCL patients, PRs and CRs were observed with single-agent oral lenalidomide. This led to an international multicenter phase 2 trial: the EMERGE study23 found an ORR of 28%, with a median PFS of 4.0 months and a median OS of 19 months in 134 heavily pretreated patients who had failed bortezomib. In the NHL-003 study, 35% of 57 relapsed MCL patients responded, with a median PFS of 5.7 months.26 Toxicity in both studies was predominantly reversible myelosuppression. Responses have been observed in patients relapsing after SCT, including the achievement of CR.
Preclinical data demonstrating synergy with rituximab has led to current testing of so-called R2 regimens in CLL and indolent lymphoma and in the Intergroup MCL trial described above. A phase 1/2 study of lenalidomide plus R found an ORR of 56%, with an 11-month PFS in 44 patients with relapsed or refractory MCL.27
mTOR inhibitors
mTOR is a downstream signaling molecule in the BCR/PI3K/AKT pathway that serves a critical role in regulating mRNA translation and interrupting cyclin D1-dependent pathways. Temsirolimus, a derivative of rapamycin, was shown in phase 2 single-agent trials to confer a 40% ORR in relapsed MCL, with higher response in combination with rituximab28 A phase 3 comparison of temsirolimus versus investigators' choice of therapy found superior ORR and PFS with temsirolimus in a heavily pretreated patient population.29 Everolimus has also shown single-agent efficacy in relapsed or refractory MCL, including patients who were refractory to bortezomib.30
BCR pathway inhibitors
Antigen stimulation of normal B cells triggers dimerization of the BCR, a transmembrane IgM antibody complex, triggering a downstream signaling kinase cascade that in turn leads to B-cell maturation, proliferation, and survival. Tonically activated BCR signaling via PI3Kδ is also a critical mechanism for normal B-cell survival. Once antigen stimulation has occurred, the dimerized BCR leads to phosphorylation of the tyrosine kinases LYN and SYK, and subsequently of Bruton tyrosine kinase (BTK), PI3Kδ, and PLCγ, each of which is necessary for BCR pathway activation.31 This pathway is constitutively activated in most B-cell lymphoproliferative disorders, making it an attractive therapeutic target.
Ibrutinib (PCI-32765), an orally bioavailable BTK inhibitor, has shown dramatic single-agent activity in MCL and CLL.32-34 The international phase 2 study of ibrutinib in 115 patients with previously treated MCL demonstrated an ORR of 66% regardless of prior bortezomib exposure.33 Treatment was well-tolerated, with only rare grade 3 or higher adverse events; the most common toxicities were grade 1-2 diarrhea and fatigue. Ibrutinib is also an inhibitor of the chemokine receptor CXCR4, leading to the mobilization of lymphoma cells into the peripheral blood from spleen, lymph nodes, and other tissues observed in many patients during initial therapy. Comparative phase 3 trials are ongoing to evaluate the efficacy of ibrutinib for MCL and other B-cell malignancies, both alone and in combination with other agents.
Idelalisib (CAL-101, GS-1101) is an orally bioavailable inhibitor of the delta isoform of PI3K, expressed in more than 90% of B-cell lymphomas. A phase 1 trial of continuous daily dosing of idelalisib in relapsed or refractory MCL showed responses in 16 of 40 patients (40%, including 2 with CR); those treated at dose levels at or above 150 mg twice daily showed a 69% ORR (11/16 patients).35 The median duration of response was 2.7 months, with a 1-year PFS of 22%. Toxicities included diarrhea, nausea, fever, fatigue, and rash, most grade 1-2, with transaminase elevations observed in most patients (20% grade 3-4). Studies are in progress evaluating idelalisib's efficacy in combination with rituximab, bendamustine, bortezomib, and everolimus.36,37 A proposed mechanism for escape from idelalisib response is the up-regulation of other PI3K isoforms, for which small-molecule inhibitors are also in clinical trial. IPI-145, a dual inhibitor of both the PI3Kδ and PI3Kγ isoforms, showed activity in a phase 1 trial of relapsed aggressive B- and T-cell lymphomas.38
Other BCR pathway inhibitors in development for numerous B-cell malignancies include the SYK inhibitor fostamatinib, as well as the PKCβ inhibitor enzastaurin. Therefore, BCR pathway inhibition represents an exciting new approach for the treatment of B-cell malignancies that is anticipated to fundamentally change the treatment approaches to these disorders in coming years.
Histone deacetylase inhibitors
Histone deacetylase (HDAC) regulates oncogenesis via modulation of transcriptional regulation of oncogenes and tumor suppressor genes. Cyclin D1 protein levels and the PI3K/AKT pathway can be down-regulated in MCL cells in vitro by treatment with vorinostat, an HDAC inhibitor currently registered for the treatment of cutaneous T-cell lymphoma. Preliminary studies of vorinostat have shown clinical responses in MCL, with further trials of this and other HDAC inhibitors in progress.39 Combinations of these agents with cytotoxics and bortezomib are currently being studied. The oral pan-HDAC inhibitor abexinostat (PCI-24781) has also shown activity in relapsed/refractory MCL and follicular lymphoma in a phase 2 trial.40 Preclinical data support the use of HDAC inhibitors in combination with proteasome inhibitors and other agents, with early clinical trials in development.
Cell cycle inhibitors
The uniform presence of cell cycle dysregulation via cyclin D1 expression has made cyclin D1 a theoretically attractive, although clinically difficult, therapeutic target. Flavopiridol is a synthetic flavone that down-regulates cyclins D1 and D3 and competitively inhibits the cyclin-dependent kinases CDK4 and CDK6. To date, however, studies have shown only modest response rates in MCL. Directly targeting CDK4/CDK6 circumvents the up-regulation of cyclin D2 or D3, which has been proposed as a mechanism of resistance after cyclin D1 inhibition. PD0332991, a selective CDK4 and CDK6 inhibitor with activity in relapsed MCL, showed an 18% ORR in preliminary analysis.41 PD0332991 is also being tested in combination with bortezomib.
Bcl-2 inhibitors/BH3 mimetics
The regulation of cell death pathways consists of both prosurvival and proapoptotic proteins, the latter characterized by the presence of a BH3 ((Bcl-2 homology 3) domain. Several BH3 mimetics are in clinical trial, including ABT-199 (GDC-0199), obatoclax (GX 15-070), and navitoclax (ABT-263). A recent phase 1 study of the potent BCL-2 inhibitor ABT-199 showed high responses in patients with relapsed MCL using single daily oral dosing, with all 8 patients achieving a PR.42 BCL-2 is highly expressed in MCL, thus rendering BH3 mimetic agents of considerable interest as single agents or in combinations that target 2 or more relevant pathways.
Summary and conclusions
Insights regarding the biologic and molecular underpinnings of MCL are continuing to be elucidated, with implications for patient prognosis, risk-adapted therapy, and the application of dose-intensive regimens and targeted agents. The long-held reputation of MCL as being among the poorest prognostic subtypes of non-Hodgkin lymphomas clearly has changed for the better as improved induction regimens, maintenance strategies, and novel agents are adapted into clinical use.
Finally, in response to the query posed at the outset, the use of a rituximab plus high-dose cytarabine-containing induction regimen followed by ASCT consolidation remains “the right thing to do” for younger and SCT-eligible patients. It is anticipated, however, that the application of high-dose therapy may decline in coming years with the increasing availability of effective, less toxic regimens and targeted agents. For older patients and those with significant comorbid illness, lower-intensity chemotherapy followed by maintenance rituximab is a standard frontline approach, with noncytotoxic regimens being tested with novel targeted agents and newer immunotherapeutics such as obinotuzumab.43 Given the relative rarity of MCL and the large number of promising therapeutic approaches to be tested, it is imperative to enroll patients on clinical trials whenever possible.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Celgene and Millennium; has received research funding from Celgene, Genentech, Janssen, Millennium, Pharmacyclics, Onyx, and Gilead; and has consulted for Celgene, Genentech, and Pharmacyclics. Off-label drug use: ibrutinib, idelalisib, lenalidomide.
Correspondence
Michael E. Williams, MD, ScM, University of Virginia Health System, P O Box 800716, Charlottesville, VA 22908; Phone: 434-924-9637; Fax: 434-243-6086; e-mail: mew4p@virginia.edu.