Abstract
Recently, a refined cytogenetic and molecular classification fundamentally changed the prognostication of patients with myelodysplastic syndromes (MDS). The increasingly complex heterogeneity of this disease entity is mirrored by life expectancy rates ranging from almost a decade for very low-risk disease down to several months in higher-risk patients, even with conventional treatments. Intensive treatment approaches are hampered by the older age of most of the patients, potentially leading to an unacceptable adverse event rate. This is especially true for allogeneic hematopoietic stem cell transplantation (HCT), which, albeit of curative intent, can lead to considerable morbidity and mortality mostly as a result of organ toxicity, infectious complications, and GVHD. Furthermore, innovative drug developments, including hypomethylating agents, have broadened the therapeutic armamentarium and, although not curative, can lead to durable responses in subgroups of patients with higher-risk MDS. In fact, there is still no prospective randomized trial available that formally demonstrates the benefit of allogeneic HCT compared with standard treatments in MDS patients. In the absence of randomized data, when considering allogeneic HCT, emphasis should be put on patient selection and optimization of the pre- and posttransplantation treatment period. In these patients, a thorough comorbidity evaluation is mandatory and stratification according to age, cytogenetics, cytopenias, disease-related quality of life, and available alternative treatments should be performed in deciding whether, when, and how to perform allogeneic HCT.
Introduction
Almost every review on myelodysplastic syndromes (MDS) starts with the important and very true premise that this term summarizes a group of heterogeneous and complex hematologic disorders primarily found within the older population, because the majority of patients are older than 60 years at diagnosis. This makes this disease challenging, not only in terms of diagnostics, but also in clinical decision making. In patients above the age of 70 years, the incidence is estimated at up to 60/100 000 per year. Indeed, the prevalence of this disease is constantly rising as a result of increasing longevity of the overall population. Many of these patients are in need of disease-specific therapies because MDS causes severe cytopenia that often manifests as RBC transfusion dependency (RBC-TD). Given the age of most MDS patients, therapeutic interventions are per se often limited to rather nonintensive treatment approaches. This article reviews the current evidence for allogeneic hematopoietic cell transplantation (HCT) as a therapeutic option in the context of disease-specific characteristics and current available alternative treatments.
General concepts of treating patients with MDS: where is the position of allogeneic HCT?
Patients with MDS are clinically subdivided into “lower-risk” (low/int-1) and “higher-risk” (int-2/high) disease according to the International Prognostic Scoring System (IPSS) risk score.
Traditionally, erythropoiesis-stimulating factors are mainly used for eligible patients in need of RBCs according to their transfusion requirement and endogenous erythropoietin level. Until recently, best supportive care was considered the primary standard treatment for higher-risk older MDS patients, whereas, supported by retrospective analyses, allogeneic HCT after myeloablative conditioning (MAC) was the standard treatment for higher-risk patients younger than 60 years of age1 with a compatible donor. Meanwhile, hypomethylating agents (HMAs) such as azacitidine (AZA) and decitabine have become the standard approach for older patients with higher-risk MDS, although they are also active in lower-risk disease. Based on a randomized study2 comparing AZA with conventional care (excluding allogeneic HCT), the drug was approved across the world and has become the standard therapy for higher-risk MDS patients. Decitabine has also been approved for MDS (according to French-American-British classification) in the United States, whereas in Europe, the label covers acute myeloid leukemia (AML) patients with 20% or more BM blasts. As yet, no randomized prospective trial has formally compared allogeneic HCT with other treatment options in patients with MDS, especially those older than 60 years of age. Nevertheless, with the introduction of reduced-intensity conditioning (RIC) and the consecutive perspective of reducing early transplantation-related mortality, the numbers of transplantations mainly in higher-risk MDS patients have consistently increased over the past decade.
The search for a donor: always successful and which graft to prefer?
Although it may seem trivial, it is important to note that the main prerequisite for performing an allogeneic HCT is to identify a suitable donor. Due to the social development and decline in the birth rate in the so called “developed countries,” matched related donors (RDs) cannot always be identified. Currently, there are more than 14 million volunteer donors registered in internationally connected registries worldwide. As a result, in ∼ 50% to 70% of all eligible patients, a matched unrelated donor (MUD) can be identified. When using high-resolution typing, the results with RDs and MUDs can be considered comparable with respect to overall and event-free survival, although the rate of chronic GVHD seems to be higher with unrelated donors. We prefer a younger MUD over a RD only in case of donor age of > 65 years, also because of recent retrospective data suggesting an improved survival with younger unrelated donors (age < 30 years) compared with older matched RDs or MUDs,3 which, however, is not supported by another study.4 Although peripheral blood stem cells (PBSCs) are widely used in MDS patients, a recent international multicenter study suggests that BM grafts instead of PBSCs might be associated with comparable long-term results after conventional MAC but may lead to less chronic GVHD.5 Because the majority of transplantations in MDS use RIC before HCT, in which a strong GVL effect is considered to be mandatory to achieve treatment success, the majority of transplantation centers still apply PBSC grafts in this setting.
Cord blood–derived hematopoietic grafts provide the advantage of transplanting rather “immature” cells, which allows successful HCT in some patients even in the presence of HLA mismatches. Nevertheless, we use cord blood transplantations in MDS patients very infrequently and prefer 2 units in cases with low cell numbers. In cases with no identifiable “conventional” donor, allogeneic HCT with BM from haploidentical family donors using posttransplantation cyclophosphamide may represent a valuable alternative solution for patients who are in need of transplantation. This technique has revolutionized transplantation modalities and potentially allows allografting for many patients with MDS.6 A recent retrospective analysis of patients with hematological malignancies, including some MDS patients, suggests that haploidentical HCT performed using T-cell-replete grafts and posttransplantation cyclophosphamide achieves outcomes equivalent to those of conventional transplantation performed using RDs and MUDs.7 Therefore, the question of having or not having a donor might not guide the physician's general judgment in the future as to whether to consider transplantation in a given MDS patient.
Risks and benefits of the procedure: what to tell the patient?
First, the patient needs to know that allogeneic HCT can, but does not necessarily, result in cure. In fact, the risk of relapse is mainly determined by disease stage and cytogenetics at the time of transplantation.8 Secondly, organ toxicities, infectious complications, and GVHD remain the major complications after HCT and can be associated with severe morbidity, reduction in quality of life, and a high rate of mortality affecting up to 30% of patients. Last but not least, in addition to many other issues, the patient needs to be counseled about alternative treatment options and their potential impact on the short- as well as long-term outcome. These variables and considerations should guide us when deciding whether to recommend a transplantation-eligible patient to undergo allogeneic HCT.
When and in whom to do the transplantation: as early or as late as possible?
Currently, patient stratification, mainly based on MDS risk scores, age, and comorbidities, is used to identify potential candidates who may benefit from allogeneic HCT. In general, the earlier the transplantation takes place during the disease course, the better the chances of long-term cure. Conversely, patients with less-advanced disease should not be exposed to the substantial risk of nonrelapse mortality (NRM) associated with this procedure.
What about disease risk?
Even though the IPSS was developed mainly to determine the prognostic risk in newly diagnosed MDS patients, its predictive value concerning posttransplantation outcome has been confirmed in several studies. According to a decision model published a decade ago,1 patients with intermediate-2 or high-risk MDS by IPSS criteria should be considered for HCT at the time of diagnosis if an HLA-matched donor is available at this time. However, this analysis was restricted to MDS patients below the age of 60 years undergoing HLA-matched sibling BM transplantation after MAC and therefore did not reflect the “true” MDS population: older patients. Furthermore, at the time of the analysis, conventional treatment options were very limited, including supportive care (eg, with antifungals). Since then, new therapeutic options, mainly with HMAs, have been introduced into the clinical setting and are able to alter the natural course of the disease.9 Nevertheless, recent retrospective analyses have suggested that allogeneic HCT in older, higher-risk MDS patients undergoing RIC is superior compared with treatment with HMAs, although the observed benefit occurred with a delay after HCT.10,11
In addition to these patient groups, those with IPSS intermediate-1 harboring adverse-risk attributes including poor-risk cytogenetics, severe thrombocytopenia, or severe RBC-TD12 might also be considered for allogeneic HCT on an individual basis. The IPSS has been revised recently to include new cytogenetic subgroups. As shown recently, the IPSS-R is associated with treatment outcome not only with supportive care,13 but also with AZA alone.14 Further, the relapse rate of patients is also significantly influenced by cytogenetics, exceeding 50% in patients with very poor-risk karyotype even after allogeneic HCT.8 This is also true for certain molecular aberrations,15 which also seem to affect the outcome of patients receiving supportive care.16 These findings show clearly that a given prognostic factor in MDS retains its significance even in the presence of therapies potentially altering the course of the disease. Therefore, treatment decisions have become challenging, further supporting the inclusion of MDS patients into clinical trials.
What about age and comorbidities?
An important question to be raised is whether the patient is in principle eligible for this procedure given his or her age and potential comorbidities. Recent large retrospective analyses of the European Group for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR) have demonstrated that (with obvious patient selection) calendar age per se is not, but performance status is, an independent risk factor for outcome after transplantation in MDS patients.17,18 In one study, the investigators found that advanced disease stage at the time of HCT was the major predictor associated with overall survival. In addition, McClune et al demonstrated that greater HLA disparity but not age adversely affected NRM and overall survival, whereas high-risk cytogenetics adversely affected survival as a result of increased risk of relapse. As a result, we consider age not to be a general barrier to allogeneic HCT and offer this option to eligible and medically fit patients up to the age of 70 years. Above this age, transplantation should be offered only to exceptionally fit patients.
Comorbidities should be assessed using the “Sorror” HCT-specific comorbidity index because multiple retrospective studies have shown its impact on NRM and overall survival in allogeneic HCT recipients apart from MDS.19 Due to the requirement of systemic immunosuppression with calcineurin inhibitors, attention needs to be drawn to a potential renal insufficiency before HCT. Recently, Deschler et al used geriatric and quality-of-life assessment instruments to develop a new prognostic score predicting HCT outcome in elderly MDS patients.20 These tools can be helpful in assessing the risk of the overall transplantation procedure given that the higher the comorbidities are, the higher the risk of NRM. Equally important as comorbidities is the clinical presentation and performance status of the patient. The main question is whether the patient is severely impaired by the disease itself (eg, RBC-TD, infections) or antecedent treatments. Patients with “symptomatic” MDS will potentially benefit from an allogeneic intervention, whereas in patients in whom quality of life is preserved despite being a transplantation candidate by disease risk, HCT could rather lead to a subsequent reduction of quality of life.
Are there therapeutic alternatives and when is a good time to proceed to HCT?
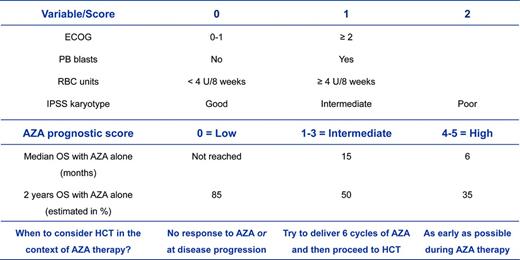
Choosing the optimal moment and integrating HCT with other therapeutic alternatives will continue to be a major challenge in daily practice. In the setting of treatment-naive patients, first-line therapies with HMAs such as AZA are considered standard of care in older patients (> 60 years of age).9 However, retrospective studies by me and others have demonstrated that allogeneic HCT might result in a survival benefit compared with AZA therapy alone in higher-risk MDS patients in their seventh decade of life.10,11 In patients undergoing single-agent treatment with HMAs, initial hematologic responses occur in ∼ 50% of patients and seem to be independent of cytogenetic risk groups, including patients with complex abnormalities.21 However, poor-risk cytogenetic abnormalities, including cases with an additional TP53 mutation9,22 are associated with lower survival rates compared with other cytogenetic subtypes. Further poor prognostic variables include the presence of peripheral blasts and severe RBC-TD,21 thus potentially allowing a prediction of outcome before the start of AZA (Figure 1). In fact, there is a subgroup of patients with normal karyotype, absence of peripheral blasts, and severe RBC-TD who might achieve long-term disease control, but not cure, with HMA alone. Given the potential and rather condensed immediate risks of allogeneic HCT, we would tend to provide transplantation to these patients outside of a clinical trial only upon disease progression with HMAs. Another reason for this is that early transplantation in patients who are considered to achieve a good response and sustained disease control with HMAs only remains questionable in the absence of data from prospective trials. In patients with an anticipated short-term benefit with HMAs only (eg, an AZA score of ≥ 1; Figure 1), allogeneic HCT should be planned as early as possible and exposition to HMA should be limited with the goal of achieving the highest potential reduction in disease burden before transplantation.
Considerations of when to proceed to an allogeneic HCT in a transplantation-eligible patient with higher-risk MDS in the context of an anticipated prior treatment with AZA according to the AZA prognostic score.21 The benefit of a therapy with single-agent AZA can be estimated according to the AZA prognostic score. As a result, one might estimate the optimal time point of when to consider proceeding to allogeneic HCT in a transplantation-eligible patient. Several trials with AZA have shown that at least 80% of patients achieved their best response after only 6 cycles of treatment. This means that only a minority of patients can further deepen their magnitude of response (eg, from partial to complete response) by the administration of additional cycles. Therefore, the continuation of AZA is considered to preserve the response already achieved at this time point. Patients with an AZA score of ≥ 1 have, in general, a high likelihood to lose their response early, even in the presence of a subsequent continuation of AZA. Therefore, I suggest limiting exposure to AZA in this group of patients in cases in which a donor has been already identified. OS indicates overall survival; PB, peripheral blood; and ECOG, Eastern Cooperative Oncology Group performance status.
Considerations of when to proceed to an allogeneic HCT in a transplantation-eligible patient with higher-risk MDS in the context of an anticipated prior treatment with AZA according to the AZA prognostic score.21 The benefit of a therapy with single-agent AZA can be estimated according to the AZA prognostic score. As a result, one might estimate the optimal time point of when to consider proceeding to allogeneic HCT in a transplantation-eligible patient. Several trials with AZA have shown that at least 80% of patients achieved their best response after only 6 cycles of treatment. This means that only a minority of patients can further deepen their magnitude of response (eg, from partial to complete response) by the administration of additional cycles. Therefore, the continuation of AZA is considered to preserve the response already achieved at this time point. Patients with an AZA score of ≥ 1 have, in general, a high likelihood to lose their response early, even in the presence of a subsequent continuation of AZA. Therefore, I suggest limiting exposure to AZA in this group of patients in cases in which a donor has been already identified. OS indicates overall survival; PB, peripheral blood; and ECOG, Eastern Cooperative Oncology Group performance status.
Most importantly, recent large analyses have shown that the survival of patients with failure to HMAs is dismal, with a median survival of < 6 months.23 In fact, only a subset of patients subsequently undergoing allogeneic HCT achieved long-term disease control afterward compared with other conventional treatment options. Therefore, especially in these patients, if eligible, allogeneic HCT should be planned as early as possible.
Therapy before allogeneic HCT: what is the role of induction chemotherapy?
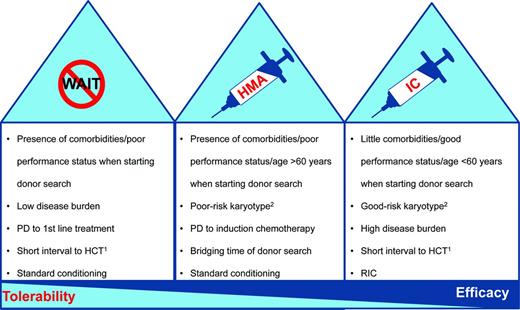
Large analyses have demonstrated improved outcomes for patients receiving HCT in complete remission compared with those with active disease at the time of HCT.24 These retrospective studies are hampered by a certain selection bias for patients with chemosensitive disease and do not take patients into consideration who did not undergo HCT because of therapy-related toxicity. Therefore, the value of prior induction chemotherapy (IC) is still not clear in the absence of randomized trials.25 In addition, IC can be associated with a considerable toxicity mainly in the absence of response. Two recent retrospective studies have demonstrated that pre-HCT therapy with AZA compared with IC may allow for similar outcomes after allogeneic HCT.26,27 Nevertheless, because the rate of complete remissions is generally higher with IC compared with HMAs, that strategy might still be the best option in selected (Figure 2) medically fit (younger) patients with a high disease burden. An important prerequisite before the initiation of IC may be the availability of a suitable donor, mainly to be able rescuing nonresponding patients.28,29 This is especially true for patients with poor-risk cytogenetics and an expected lower response rate compared with other karyotypic abnormalities.
Considerations for choosing the optimal treatment before allogeneic HCT in patients with MDS. In general, there are 3 potential treatment options for transplantation-eligible patients before allogeneic HCT. The figure provides some rationale for choosing the optimal therapy before a planned transplantation. PD indicates progressive disease. 1Donor already identified. 2In general, IC can achieve higher complete response rates than AZA irrespective of karyotype abnormalities. The recommendation above is based on the fact that patients with a poor-risk karyotype have a lower chance to respond to IC than patients with normal cytogenetics (∼ 40% vs 70%). In patients with poor-risk karyotype and no identified donor, a soft “bridging” (although with a lower chance of response than with IC) that avoids the immediate toxicities of IC might be a reasonable alternative. Alternatively, patients with a good-risk karyotype have a good chance of responding to IC, which might therefore be considered as an option even in the immediate absence of a compatible donor.
Considerations for choosing the optimal treatment before allogeneic HCT in patients with MDS. In general, there are 3 potential treatment options for transplantation-eligible patients before allogeneic HCT. The figure provides some rationale for choosing the optimal therapy before a planned transplantation. PD indicates progressive disease. 1Donor already identified. 2In general, IC can achieve higher complete response rates than AZA irrespective of karyotype abnormalities. The recommendation above is based on the fact that patients with a poor-risk karyotype have a lower chance to respond to IC than patients with normal cytogenetics (∼ 40% vs 70%). In patients with poor-risk karyotype and no identified donor, a soft “bridging” (although with a lower chance of response than with IC) that avoids the immediate toxicities of IC might be a reasonable alternative. Alternatively, patients with a good-risk karyotype have a good chance of responding to IC, which might therefore be considered as an option even in the immediate absence of a compatible donor.
The use of HMAs instead of IC for the reduction of disease burden has changed potential strategies in preparation for HCT.30,31 Treatment with HMAs is feasible and mostly well tolerated and should be considered mainly for older (> 60 years) and “comorbid” patients and as a “bridging strategy” to HCT in those in whom no donor has yet been identified. In addition, several predictive factors for long-term outcome with HMAs have been determined (Figure 1) and might therefore guide treatment decisions regarding when to finally proceed to transplantation.21
Which conditioning before transplantation?: is RIC better than MAC?
Up to the early 1990s, standard conditioning regimens with myeloablative intent (MAC) were used in patients with MDS and had at least in part contributed to the excess of NRM in these patients. Because the intensity of transplantation conditioning is linked to NRM, the development of RIC regimens and the use of alternative donor sources have allowed the successful application of HCT in older and comorbid patients with MDS as well. Conversely, RIC transplantations rely on the GVL effect and have been associated with a higher risk of disease relapse compared with conventional conditioning.17,18 Compounds commonly used in RIC regimens are fludarabine combined with low-dose total body irradiation or busulfan and treosulfan, but no regimen has been formally shown to be superior compared with others. The results of these studies have been summarized in several recent reviews.32,33 Nevertheless, MAC regimens are still considered in certain circumstances, mainly in younger and medically fit MDS patients (Figure 2). A recent study by our group in AML patients < 60 years of age in remission demonstrated less toxicity with RIC compared with standard conditioning with similar survival outcomes.34 Furthermore, the EBMT has recently completed a prospective study (www.clinicaltrials.gov identifier NCT00682396) comparing RIC versus MAC in younger MDS patients and the final results are awaited.
RBC transfusion dependency: is iron a culprit or a bystander?
A large body of evidence exists from retrospective studies showing that systemic iron overload (SIO) in MDS patients (mainly as a result of RBC-TD before HCT) is associated with increased risk of early mortality after allogeneic HCT.12,35 The reasons for this observation remain, at least in part, not very well defined. Given the limitation of serum ferritin measurement, including its association with variables important for transplantation outcome such as comorbidities,12 noninvasive MRI, or detection of labile plasma iron, it is currently the most promising method to evaluate SIO and its potential toxic metabolites. In fact, we and others36,37 have recently presented data demonstrating that MRI-based liver iron concentration rather than ferritin is of prognostic significance after allogeneic HCT. Labile plasma iron is released as a result of pretransplantation conditioning; however, so far, the direct consequences of this observation in vivo and on the posttransplantation period are largely unknown. Nevertheless, treatment approaches to prevent severe iron overload are reasonable and warranted. It is recommended to use iron chelation before HCT in selected patients with SIO, although no definitive cutoff for ferritin or liver iron has been systematically defined. Alternatively, allogeneic HCT should be performed earlier, before SIO becomes clinically evident.
Relapse after allogeneic HCT: who is at risk and how to prevent it
Relapse still remains a major challenge in the care of patients after allogeneic HCT, also due to the wide application of RIC transplantations.17,18 In particular, patients who are not in remission at the time of HCT often experience disease recurrence. Therefore, the main factors influencing relapse risks after transplantation are disease burden before HCT (reflected by blast count and RBC-TD) and cytogenetic risk group. In fact, in a recent publication by the Seattle group,8 a rather dismal outcome (with a relapse rate of > 50%) has been reported, especially for patients with “very poor” risk cytogenetics including monosomal karyotype according to the revised IPSS classification.13 Therefore, these patients need to be carefully evaluated for their curative potential with an allogeneic HCT to determine whether they should be exposed to the immediate hazards of the procedure or if alternative treatment options exist.
Generally, the prognosis of MDS patients relapsing after allogeneic HCT is poor, especially in the case of an early relapse within the first 6 months after HCT because patients are still recovering from the sequelae of the overall approach. At this time, further intensive therapeutic interventions often result in excessive NRM. The optimal treatment strategy for MDS patients relapsing after HCT also remains undefined because prospective trials comparing different approaches are lacking. Several therapeutic approaches to controlling disease, including withdrawal of immunosuppression, donor lymphocyte infusions, chemotherapy, and a second HCT, are available but are often not successful or result in severe toxicity. In particular, patients with poor-risk cytogenetics, overt disease, and a short interval (< 1 year) from the time of allogeneic HCT have a low likelihood of achieving a second long-term remission.
Recently, AZA has been investigated in the treatment of relapsed MDS or AML patients after allogeneic HCT.38 This study demonstrated an overall response rate of 30% that was durable in 5 of 30 patients. Apart from the direct antiproliferative and cytotoxic effects on leukemic cells, AZA might also influence the donor immune system, thereby enhancing the GVL effect.39 Therefore, the combination of HMAs and donor lymphocyte infusion seems to be an interesting treatment approach in relapsed patients. Considering the limited treatment opportunities in MDS patients relapsing after HCT, prevention of disease recurrence after HCT should be one of the major goals for future clinical research.40 Because relapse occurs predominantly within the first year after HCT, a potential maintenance therapy should start as early as possible. Due to its tolerability, AZA can be administered on an outpatient basis, which is an ideal prerequisite for this kind of approach. In fact, a recent study confirmed that early posttransplantation maintenance therapy with AZA is feasible without serious side effects and without increased of the rate of GVHD,41 which has been the backbone for a randomized trial comparing AZA versus current standard of care after allogeneic HCT (www.clinicaltrials.gov identifier NCT00887068). Another potentially appealing drug for maintenance therapy is lenalidomide, mainly in case of del(5q) disease. However, the results of a recent study in MDS and AML patients40 and in patients with multiple myeloma have shown an increased risk of GVHD.
In general, during maintenance therapy, there will be a subset of MDS patients who will never relapse after HCT and will thus unjustifiably be exposed to the potential risks and adverse events of drug treatment. The optimal strategy to circumvent this situation is a MRD-guided therapy, which offers treatment to patients with detectable MRD only after HCT. Until recently, the majority of patients with MDS often lacked a disease-specific molecular marker for MRD detection. In these patients, chimerism analyses have remained an important diagnostic tool to monitor the success of HCT. Our group has recently reported the first trial evaluating the efficacy of a preemptive treatment with AZA for MRD defined by a decreasing CD34+ donor chimerism to prevent or delay hematologic relapse in patients with CD34+ MDS or AML after allogeneic HSCT.42 Flow cytometry–based detection of MRD represents an additional tool for interventions.43 In the near future, it can be anticipated that mutational analyses will be used increasingly for MRD-guided approaches.
Summary
Should allogeneic HCT be a potential curative option in older patients with MDS? Yes, but the risks of the underlying disease have to be balanced against comorbidities, hazards of the allogeneic procedure, patient's preferences, and therapeutic alternatives. If possible, these patients should be treated within prospective trials investigating the outcome of allogeneic HCT compared with conventional treatment options with quality of life as a secondary end point. In the absence of these studies, a careful individual selection should be done. Most importantly, allogeneic HCT in its current form needs to be further developed and improved, including finding a method for better prevention of GVHD. Additional attention should be drawn to the monitoring and treatment of MRD using allogeneic HCT as a platform for MDS-specific interventions. Hopefully, all of these efforts will help to better identify the subgroup of MDS patients who will most benefit from this approach.
Acknowledgments
The author thanks Dr Martin Bornhäuser (Dresden), Dr Johannes Schetelig (Dresden), Dr Katharina Götze (Munich), and Dr Lionel Ades (Paris) for their helpful comments. Many thanks also to S. Helas for assistance in formatting the figures and to S. Faber from EMSCO (www.emsco.eu) for critically reading the manuscript.
Disclosures
Conflict-of-interest disclosure: The author has received research funding and honoraria from Celgene and Novartis. Off-label drug use: None disclosed.
Correspondence
Uwe Platzbecker, Universitätsklinikum Carl-Gustav-Carus, Medizinische Klinik I, Fetscherstraße 74, 01307 Dresden, Germany; Phone: 49351/4582583/4373; Fax: 49351/4582583/4373; e-mail: uwe.platzbecker@uniklinikum-dresden.de.