Abstract
There have been major advances in the past decade in the continuum of therapy for transplantation-eligible multiple myeloma patients. For patients requiring therapy, recommended induction treatment consists of triple drug regimens followed by the collection of hematopoietic stem cells. The question of early versus delayed transplantation is under investigation and may identify patients for whom early transplantation is optimal therapy and those for whom it may be delayed. For transplantation-eligible patients, high-dose melphalan remains the standard regimen. After transplantation, consolidation can be considered for patients with less than a complete remission. Maintenance therapy with bortezomib or lenalidomide (or both in very-high-risk patients) is a reasonable option for long-term disease control and improvement in overall survival. Incorporation of new agents into the continuum of multiple myeloma care should result in improved outcomes and long-term disease control.
Introduction
Multiple myeloma (MM) is a malignant plasma cell proliferation that occurs within a spectrum of diseases that includes monoclonal gammopathy of undetermined significance, primary amyloidosis, nonsecretory myeloma, and solitary plasmacytoma.1 MM therapy is based on related organ or tissue involvement that leads to requirement for therapy. CRAB (which stands for “hyperCalcemia, Renal failure, Anemia, and Bone disease”) criteria are the most common reasons for initiating therapy. Other indications include symptomatic hyperviscosity, recurrent bacterial infections, and amyloidosis with organ involvement. We review here the continuum of treatment of the transplantation-eligible MM patient, including induction therapy, hematopoietic stem cell transplantation (HSCT), and posttransplantation consolidation and maintenance.
Induction regimens for transplantation-eligible patients are designed to decrease the malignant plasma cell burden and improve the depth of response.2 Depth of response after autologous transplantation appears to correlate with the duration of disease control before disease progression occurs with the need for salvage therapy.3 Various strategies have been undertaken to control disease and improve outcome in MM patients.4 In addition to depth of response, other prognostic criteria influence long-term outcome. These include disease staging (β2-microglobulin and albumin levels); serum lactate dehydrogenase; cytogenetic analysis by FISH for t(4;14), t(14;16), del(17p), del(1q), and +1q; and plasma cell leukemia at presentation.5-7 Table 1 describes risk factors that allow the clinician to develop strategies for MM induction, transplantation, consolidation, and maintenance treatment. These risk factors will change over time as new agents are developed, which will change risk stratification. As an example, the del(13) cytogenetic abnormality by metaphase karyotyping was considered high risk before the widespread availability of bortezomib, which reduces this risk.4-7 Molecular risk stratification currently by gene expression profiling (GEP) is becoming an important tool for identifying very-high-risk patient populations.8,9 However, there are currently no approved treatment approaches to control very-high-risk disease. GEP and other molecular techniques, including next-generation sequencing and single nucleotide polymorphism arrays, are future strategies that could facilitate the systematic incorporation of new therapies into the continuum of MM treatment. The development of new diagnostic approaches combined with novel treatments may allow for the maintenance of long-term disease control and improved outcomes for all MM patients.
High-risk factors at diagnosis

Cytogenetics: del(13) is high risk by metaphase karyotyping. Recommend that FISH be performed after CD138 selection of BM cells to increase plasma cell yield. GEP 70 is validated; EMC-92 has early validation and is ongoing. New molecular genetic tests await validation in multiple datasets.
LDH indicates lactate dehydrogenase; EMC, Erasmus Medical Center; and GEP, gene expression profile.
Induction regimens
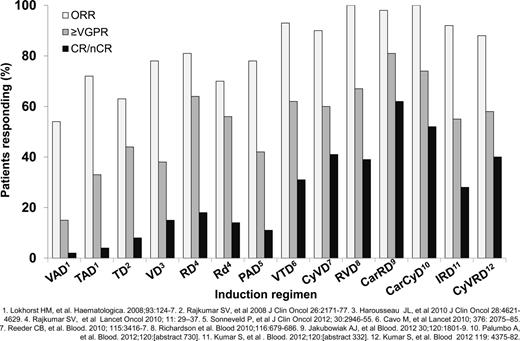
Induction regimens for MM patients requiring therapy have improved over the last decade. A recent report updated the continued improvement in survival for transplantation-eligible and transplantation-ineligible MM patients over the past 10 years.10 Induction regimens for transplantation-eligible MM patients have improved since the introduction of high-dose dexamethasone in combination with doxorubicin and vincristine (for review, see Ludwig et al4 ). Immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) were first used as salvage agents, then later as part of combination induction therapy. The combination of an IMiD with a PI along with dexamethasone has resulted in marked improvements in disease response. Improved depth of response has resulted in improved progression-free survival (PFS) and overall survival (OS). Figure 1 shows the increasing overall response (≥ partial response) rate, the very good partial response rate (VGPR), and near complete (nCR)/complete response (CR) rates with the incorporation of new agents.11-22 Increasing the frequency and depth response after induction followed by autologous HSCT has led to improved outcomes.23 Different strategies have been used to improve PFS. Most standard induction regimens contain 3 drugs and have included an IMiD or PI and increasing frequency; both drugs are used in combination with dexamethasone. Attempts to increase to 4 or more agents have not yet resulted in improved responses due to increased toxicity.22 A philosophical issue is the role of treatment to achieve a response plateau: to reserve future therapy for disease progression versus treatment to achieve the deepest response up-front and then maintaining that response.24-26 The principle of continued therapy with minimal to no interruption applies to both transplantation-eligible and transplantation-ineligible patients.25,26 There have been no phase 3 studies comparing these approaches, but there will be opportunities for examining different strategies for MM disease control. At present, attaining a CR and maintaining this response continues to be a reasonable approach to maintain disease control and improve OS based on different strategies.3,27,28 Previous work has also demonstrated that attaining ≥ VGPR predicts for long-term disease control, yet attaining and sustaining CR has been associated with increased OS.29,30 Therefore, in responding patients, treatment with a fixed number of induction cycles or treatment until best response are common strategies. Based on the published literature, treatment until best response may be the optimal approach. In the absence of response after 1-2 cycles of induction, changing to another regimen to attain ≥ VGPR is a standard approach because treatment with an autologous HSCT without disease control usually results in short-term responses.
HSCT
Autologous HSCT
The transplantation-eligible patient may be defined by age and/or comorbidities. In some countries, most patients over the age of 65 years do not undergo autologous HSCT. In the United States, age over 65 years is not considered an absolute contraindication and other factors, especially comorbidities and performance status, influence the decision for a patient to undergo an autologous HSCT. Autologous HSCT has become the standard approach to improve or deepen response and can be considered a form of consolidation. We will use the term consolidation for the period of intensification therapy after HSCT. After attaining optimal response, peripheral blood stem cells (PBSCs) are collected for at least one, and usually more than one, autologous HSCT. Three collection approaches include chemotherapy mobilization with growth factor support (G-CSF), G-CSF with plerixafor, or G-CSF alone. A second transplantation could be offered to the MM patient who relapses after at least 12-24 months after the first autologous transplantation; collecting PBSCs early in the course of treatment will diminish exposure to potentially mutagenic therapies. Double or tandem autologous HSCT has been shown to be superior to single autologous HSCT in patients who do not attain at least a VGPR.31 It is not certain whether tandem autologous HSCT up-front is superior to a single transplantation followed by second autologous HSCT after disease progression. With the use of novel agents and consolidation after transplantation, new studies will help to determine the role of tandem autologous HSCT. There has been one phase 3 study examining induction followed by up-front autologous HSCT compared with rescue autologous HSCT at disease progression/relapse, which found no difference in the 2 approaches.32 Because this study was reported more than 10 years ago, ongoing studies incorporating newer agents will help to identify patients benefiting most from early autologous HSCT. Two early reports of phase 3 trials examining chemotherapy versus up-front tandem autologous HSCT have shown an improved PFS without a difference in OS.33,34 One examined 402 newly diagnosed MM patients receiving Rd (lenalidomide, dexamethasone) induction followed by randomization to MPR (melphalan, prednisone, lenalidomide) or tandem autologous HSCT with melphalan at 200 mg/m2 (Mel200) with each transplantation. All patients were then randomized to lenalidomide maintenance until progression.33 At a median follow-up of 45 months from diagnosis, the median PFS for the tandem Mel200 arm was significantly superior to the MPR arm (38 vs 26 months, P < .0001). The second study examined 389 newly diagnosed MM patients who received Rd induction followed by randomization to CRD (cyclophosphamide, lenalidomide, dexamethasone) or tandem autologous HSCT with Mel200. A second randomization occurred to lenalidomide/prednisone versus lenalidomide maintenance until progression.34 The 2-year PFS was 72% for the Mel200 arm and 61% for the CRD arm, (P < .001). These 2 studies did not use bortezomib during induction or maintenance and longer follow-up will be necessary to determine whether there will be a difference in OS. Other phase 3 studies are ongoing that will help define the role of autologous HSCT, consolidation, and maintenance therapies in the long-term disease control of MM. These are described later in this chapter. Autologous HSCT dose-intensive regimens consist of high-dose melphalan (Mel200). So far, no other regimen either substituting for or adding to melphalan has been shown to be consistently superior to high-dose melphalan alone.
Allogeneic HSCT
Allogeneic HSCT is not clearly established as a standard treatment approach for most MM patients, and enrollment in a clinical protocol when available for high-risk patients is recommended. A recent meta-analysis evaluated 6 trials comparing tandem autologous HSCT versus autologous HSCT followed by allogeneic reduced intensity conditioning (RIC) HSCT.35 Allogeneic RIC after autologous HSCT was associated with a higher CR rate and treatment-related mortality (TRM) without a clear benefit in PFS and OS. Study design, length of follow-up, and toxicity issues influenced this analysis. Very-high-risk younger patients, such as those with a high lactate dehydrogenase, plasma cell leukemia, del(17) cytogenetic mutations, or high-risk GEP-70, could be considered for allogeneic transplantation. However, early trials with myeloablative allogeneic HSCT were limited by a high TRM rate and the majority of patients relapsing after tandem autologous/RIC allogeneic HSCT. Therefore, reduction of TRM and improvement of long-term outcomes through novel strategies such as consolidation and maintenance are necessary before allogeneic HSCT can be widely offered for high-risk MM patients.
Consolidation after autologous HSCT
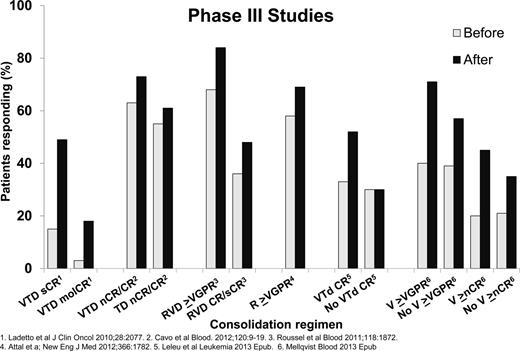
Consolidation is a short course of therapy designed to intensify and deepen response to initial therapy. Consolidation therapy for MM patients consists of different forms of intensification after single or tandem autologous HSCT. Figure 236-41 shows the improvement of response with consolidation therapies after single or tandem autologous HSCT. All studies have shown an improvement in response rates after a short course of consolidation after autologous HSCT. The Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) Italian Myeloma Network phase 3 study of 480 patients examined induction therapy with VTD (bortezomib, thalidomide, and dexamethasone) versus thalidomide and dexamethasone (TD), followed by tandem autologous HSCT with Mel200 followed by 2 cycles of VTD consolidation for the VTD induction arm or 2 cycles of TD consolidation for the TD induction arm. After VTD consolidation, the CR rates (61% vs 47%, P = .012) and CR/nCR rates (73% vs 61%, P = .020) were significantly higher for VTD versus TD consolidation therapy. There was no OS benefit at the last study report.37 A second phase 3 study of 370 patients examined 20 weekly doses of bortezomib consolidation versus no consolidation therapy. This study showed a PFS advantage (27 vs 20 months, P = .05) for the bortezomib consolidation group without an OS benefit.41 There was significant improvement in ≥VGPR rates (71% vs 47%, P < .01) and an improvement in CR/nCR rates (45% vs 35%, P = .055). There was no OS advantage with bortezomib consolidation at the time of this report. The other studies, which are not phase 3, demonstrated improvements in molecular response with VTD after tandem autologous HSCT, improvement in response with RVD (lenalidomide, bortezomib, dexamethasone) consolidation after RVD induction and single autologous HSCT, improvement in response with lenalidomide consolidation after single or tandem autologous HSCT, or improvement in response and PFS with VTD consolidation after single autologous HSCT compared with a nonrandomized cohort of patients not receiving consolidation after single autologous HSCT.36,38-40 Consolidation is more widely used outside the United States. An ongoing Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) phase 3 study examining RVD consolidation is described later in this chapter. Consolidation therapy can be considered in patients who achieve less than a CR after single autologous HSCT.
Maintenance after autologous HSCT
MM is a disease that relapses/progresses in most patients. Therefore, several studies have examined the role of several types of maintenance, including IFN, glucocorticoids, and oral melphalan to improve response and outcome (for review, see McCarthy et al42,43 ). These agents were limited in efficacy and/or tolerability. Maintenance therapy should be easy to deliver, convenient for the patient, have modest toxicity, and improve PFS and ideally OS compared with retreatment at relapse.44 Thalidomide maintenance is limited by toxicity, although prolonged use (at least 1 year) has been shown to improve PFS and OS in selected studies (Table 2).45-52 It appears to have the most benefit in MM patients with standard-risk disease and may be more efficacious when combined with glucocorticoids or bortezomib (see next section).
Thalidomide maintenance after autologous HSCT
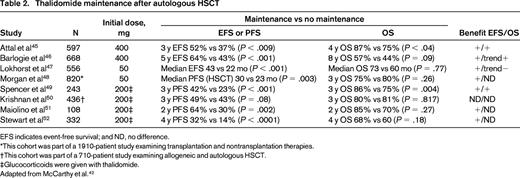
EFS indicates event-free survival; and ND, no difference.
*This cohort was part of a 1910-patient study examining transplantation and nontransplantation therapies.
†This cohort was part of a 710-patient study examining allogeneic and autologous HSCT.
‡Glucocorticoids were given with thalidomide.
Adapted from McCarthy et al.42
Three phase 3 studies, 2 examining bortezomib maintenance and 1 examining zoledronate as part of long-term maintenance/supportive care, are summarized in Table 3.15,53,54 Bortezomib maintenance after autologous HSCT has been reported to improve both PFS and OS, the latter primarily in high-risk cytogenetic patients [del(13), t(4;14), del(17)] in the Hemato-Oncologie voor Volwassenen Nederland (Dutch-Belgian Cooperative Trial Group) 65/German-Speaking Myeloma Multicenter Group-HD4 (HOVON-65/GMMG-HD4) study.15 Bortezomib was part of the induction and maintenance arms and was compared with VAD (vincristine, doxorubicin, dexamethasone) induction and maintenance thalidomide. The improvement in PFS and OS in that study could have been related to the use of bortezomib during induction, because the induction regimen, PAD (bortezomib, doxorubicin, dexamethasone), generated superior responses to VAD. Interestingly, in a subset analysis, patients receiving a tandem autologous HSCT (in Germany) had an improved PFS compared with patients receiving a single autologous HSCT (in The Netherlands). The Spanish PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) GEM (Grupo Español de MM) studied VTD for induction and found it superior to other chemotherapy regimens, including TD.53 Patients were further randomized to VT or 2 arms using T and IFN as single agents. The VT arm had a superior PFS compared with the other arms, but primarily in cytogenetically low-risk patients. This difference with the HOVON-65/GMMG-HD-4 study could be due to the intensity of bortezomib treatment (52 doses over 2 years vs 48 doses over 3 years). Bisphosphonates as supportive therapy during induction with and without autologous HSCT and continued during maintenance has been found to decrease skeletal-related events and, interestingly, zoledronate use was associated with improved OS, as reported by the Medical Research Council United Kingdom (MRC-UK) in the MRC IX MM trial.54 The reason for this finding may be due to an anti-MM effect of bisphosphonates, but an exact mechanism remains uncertain.
Bortezomib and zoledronate maintenance after autologous HSCT

SC indicates subcutaneous; IT, intensive therapy; NIT, nonintensive therapy; MV, multivariate; and ND, no difference.
Adapted from McCarthy et al.42
Lenalidomide maintenance after autologous HSCT has been reported in 3 studies (Table 4), all of which showed a PFS or time to progression (TTP) benefit and one showing an OS benefit.39,55,56 The CALGB 100104 study examined 462 MM patients after single autologous HSCT who were randomized to lenalidomide 10 mg daily (dose range 5 to 15 mg) or placebo until disease progression. Induction therapy was not mandated by protocol; however, the majority of patients received an IMiD-based regimen (74% of patients received thalidomide or lenalidomide-based induction). There was no postautologous HSCT consolidation. The median TTP was 46 months for the lenalidomide arm and 27 months for the placebo arm (P < .001). At a median follow-up of 34 months, the 3 year OS rates were 88% for the lenalidomide arm and 80% for the placebo arm (P = .028). The study had been un-blinded 22 months before this reported analysis, and 86 of 128 (67%) of eligible placebo arm patients crossed over to receive lenalidomide. A TTP and OS benefit for the lenalidomide arm remained despite the crossover. There was an increased incidence of second primary malignancies (SPMs) in lenalidomide arm compared with placebo (3.5% vs 0.4% hematologic malignancies and 4.3% vs 2.1% solid tumors). The cumulative incidence risk of SPMs was greater for the lenalidomide compared with the placebo arm (P < .008). The cumulative incidence risk of progressive disease (P < .001) or death (P < .002) was greater for the placebo arm than for lenalidomide arm. All patients benefited from lenalidomide maintenance regardless of remission status or prior exposure to thalidomide or lenalidomide during induction. In particular, patients who received lenalidomide-containing induction followed by lenalidomide maintenance had improved TTP. A new analysis was conducted and presented at the International Myeloma Workshop (IMW) in the spring of 2013. The TTP, PFS, and OS benefit were still present on an intent-to-treat analysis despite the crossover by the majority of the placebo arm patients.56 The IFM 05-02 trial randomized 605 MM patients to lenalidomide 10 mg daily (dose range, 5-15 mg) versus placebo until progression after single (79%) or tandem autologous HSCT (21%) and a 2-cycle consolidation with lenalidomide for all patients before randomization. The induction regimens were VAD or VD with almost no IMiD exposure. Approximately 25% of patients received pre-autologous HSCT consolidation with dexamethasone, cyclophosphamide, etoposide, and cisplatin. The median PFS was 41 months for the lenalidomide arm and 23 months for the placebo arm (P < .001). The 4-year PFSs was 43% for the lenalidomide arm and 22% for the placebo arm (P < .001). The OS was 74% for the lenalidomide arm and 76% for the placebo arm and the 4-year OS rates were 73% for the lenalidomide arm and 75% for the placebo arm (P = .7) at a median follow-up of 45 months. The study was unblinded at ∼22 months before the above analysis, and all maintenance was stopped at a median of ∼ 32 months of maintenance for the lenalidomide arm. Placebo arm patients did not cross over to lenalidomide maintenance at unblinding. There was an increased incidence of SPMs in the lenalidomide arm compared with placebo (4.2% vs 3.3% hematologic malignancies and 1.3% vs 2.1% solid tumors). Lenalidomide maintenance was found to benefit all patient subclassifications, including cytogenetic risk and remission status, in the IFM 05-02 study. The third lenalidomide maintenance study of 402 patients has been reported in abstract form and, in addition to comparing maintenance versus no maintenance, compared chemotherapy versus tandem autologous HSCT.33 The median PFS was 37.5 months for lenalidomide maintenance and 25.7 months for no maintenance (P = .0008). The 4-year OS from diagnosis was 76% for maintenance and 68% for no maintenance (P = .08) and the 3-year OS from maintenance initiation was 81% for maintenance and 72% for no maintenance, respectively (P = .04). There was no difference in SPM rates between the maintenance and no maintenance arms.
Lenalidomide maintenance after autologous HSCT
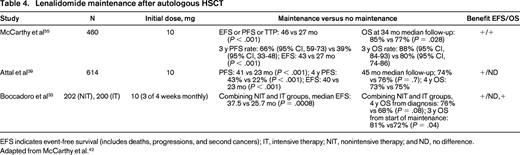
EFS indicates event-free survival (includes deaths, progressions, and second cancers); IT, intensive therapy; NIT, nonintensive therapy; and ND, no difference.
Adapted from McCarthy et al.42
Recommendations and strategies for long-term disease control
Table 557 lists current treatment recommendations for MM. Risk stratification at diagnosis will help with selecting treatment and predicting response. Patients with very-high-risk features such as del(17p) may be considered for up-front investigational approaches to long-term disease control.
Treatment recommendations for transplantation-eligible patients
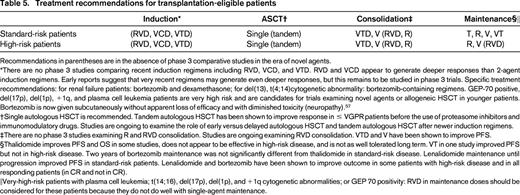
Recommendations in parentheses are in the absence of phase 3 comparative studies in the era of novel agents.
*There are no phase 3 studies comparing recent induction regimens including RVD, VCD, and VTD. RVD and VCD appear to generate deeper responses than 2-agent induction regimens. Early reports suggest that very recent regimens may generate even deeper responses, but this remains to be studied in phase 3 trials. Specific treatment recommendations: for renal failure patients: bortezomib and dexamethasone; for del(13), t(4;14)cytogenetic abnormality: bortezomib-containing regimens. GEP-70 positive, del(17p), del(1p), +1q, and plasma cell leukemia patients are very high risk and are candidates for trials examining novel agents or allogeneic HSCT in younger patients. Bortezomib is now given subcutaneously without apparent loss of efficacy and with diminished toxicity (neuropathy).57
†Single autologous HSCT is recommended. Tandem autologous HSCT has been shown to improve response in ≤ VGPR patients before the use of proteasome inhibitors and immunomodulatory drugs. Studies are ongoing to examine the role of early versus delayed autologous HSCT and tandem autologous HSCT after newer induction regimens.
‡There are no phase 3 studies examining R and RVD consolidation. Studies are ongoing examining RVD consolidation. VTD and V have been shown to improve PFS.
§Thalidomide improves PFS and OS in some studies, does not appear to be effective in high-risk disease, and is not as well tolerated long term. VT in one study improved PFS but not in high-risk disease. Two years of bortezomib maintenance was not significantly different from thalidomide in standard-risk disease. Lenalidomide maintenance until progression improved PFS in standard-risk patients. Lenalidomide and bortezomib have been shown to improve outcome in some patients with high-risk disease and in all responding patients (in CR and not in CR).
‖Very-high-risk patients with plasma cell leukemia; t(14;16), del(17p), del(1p), and +1q cytogenetic abnormalities; or GEP 70 positivity: RVD in maintenance doses should be considered for these patients because they do not do well with single-agent maintenance.
Induction
There have been no phase 3 studies that have compared newer induction regimens including RVD, VCD, and VTD. Based on phase 2 results, RVD, VCD, and possibly VTD appear to generate deeper responses than 2-drug regimens such as VD or RD/Rd. Therefore, a 3-drug regimen is recommended for induction therapy. VTD may not be as well tolerated due to neuropathy. Replacing IV administration of bortezomib with subcutaneous bortezomib as part of induction therapy has been shown to decrease neuropathy without any impact on outcomes.57 Treatment should be to best response, followed by autologous PBSC collection.
Autologous HSCT
Up-front autologous HSCT is recommended because there is a PFS benefit for early transplantation based on one phase 3 study. However, there are no data suggesting that delayed autologous HSCT would be detrimental, and autologous HSCT after first disease progression is another treatment option. A single autologous HSCT is recommended, although patients achieving less than a VGPR to initial therapy could be considered for either consolidation therapy or a second transplantation to deepen response. Two ongoing trials will help to update and refine the evidence for up-front versus delayed autologous HSCT and help to determine which patient populations should proceed to autologous HSCT and which patient populations can defer transplantation. The Dana Farber Cancer Institute trial in conjunction with the IFM will examine the role of early versus late autologous HSCT after up-front induction therapy.58 The IFM group has completed accrual and patients will receive lenalidomide maintenance for 1 year. The U.S. study will allow lenalidomide maintenance until progression. This will provide an indirect comparison of the effect of 1-year versus long-term lenalidomide maintenance on outcome. The European Myeloma Network (EMN) will examine the role of chemotherapy versus single versus tandem autologous HSCT.59 All 3 arms will be followed by a randomization to consolidation followed by maintenance versus direct maintenance therapy. In all arms, the maintenance of lenalidomide 3 weeks per month is given until progression.
Consolidation
Consolidation is primarily used outside the United States, but can be considered for patients who do not achieve a VGPR or better after autologous HSCT. PFS is improved with VTD or V consolidation. It remains to be determined whether RVD consolidation will improve outcome after single autologous HSCT. The BMT-CTN 0702 is a phase 3 study examining a single autologous HSCT followed by a second transplantation or RVD consolidation or no further intensive therapy. All 3 arms are followed by 3 years of lenalidomide maintenance therapy.60 This study should help to confirm whether improvement in CR rates leads to improved PFS and OS. The EMN-02 study is also investigating the role of RVD consolidation after chemotherapy and single or tandem transplantation.59
Maintenance
Thalidomide with or without glucocorticoids has been shown to improve PFS, and in some cases OS. However, thalidomide maintenance therapy has neuropathic side effects that can seriously affect quality of life, so most patients cannot stay on maintenance therapy even in low doses. Therefore, bortezomib or lenalidomide are recommended as the primary agents to be considered for long-term maintenance. Bortezomib may be considered for 2 years after autologous HSCT based on the HOVON-65/GMMG-HD4 trial and may be best when using a bortezomib-containing induction regimen. There has been no demonstrated increase in SPMs with bortezomib maintenance. Lenalidomide maintenance until disease progression was shown to improve PFS and OS in the CALGB 100104 study, especially in patients receiving lenalidomide-based induction and in CR and non-CR patients. The IFM 05-02 study, while not showing an OS benefit at this time, showed a PFS benefit for all patients, including high-risk patients. The cumulative incidence risk for SPM is higher in the patients receiving lenalidomide maintenance and that for progression and death is higher in patients not on maintenance therapy in the CALGB 100104 study. The risk of SPM development should be evaluated within the context of the risk of disease progression and death due to MM. There is no compelling phase 3 data that would guide maintenance recommendations for very-high-risk disease. Therefore, for very-high-risk patients with plasma cell leukemia; t(14;16), del(17p), del(1p), and +1q cytogenetic abnormalities; or GEP 70 positivity, combined bortezomib and lenalidomide maintenance therapy can be considered.
Summary and future directions
The treatment of the MM patient has improved over the past 10 years, with median PFS approaching 4 years after autologous HSCT. New agents based on MM cell metabolic pathways of growth and cell development and antibodies that have activity with current agents or single-agent activity will be tested for salvage treatment of relapsed and refractory disease.61 Several promising agents will be tested as part of induction and can be added to bortezomib or lenalidomide maintenance therapy after transplantation. Such agents include novel PIs such as marizomib and the oral PIs ixazomib and oprozomib, the recently Food and Drug Administration (FDA)–approved PI carfilzomib, the recently FDA-approved IMiD pomalidomide, the deacetylase inhibitors, the novel alkylators including bendamustine and melphalan-flufenamide, the spindle kinase protein inhibitor Arry-520, kinase inhibitors, and heat shock protein inhibitors. Other forms of immunotherapy include the monoclonal antibody elotuzumab (anti-CS1), which has activity when combined with lenalidomide, dexamethasone, and daratumumab (anti-CD38), which has single-agent activity. Antibodies to Activin A, BAFF, CD40, CD56, CD74, CD138, DKK-1, IL-6/R, RANKL, TRAIL, and VEGF/R, alone, conjugated to a cytotoxic agent, or in combination with an IMiD or PI are in clinical development. Other strategies are ongoing to incorporate vaccination against MM antigens, along with immunomodulatory agents such as IMiDs or the anti-PD-1 antibody. The incorporation of new agents into the treatment of MM patients should lead to further prolongation of response and long-term control of the disease.
Disclosures
Conflict-of-interest disclosure: P.L.M. has consulted for Celgene and has received honoraria from Celgene and Janssen. T.H. has equity ownership in Novartis. Off-label drug use: thalidomide, bortezomib, or lenalidomide for maintenance therapy in transplantation-eligible patients; bortezomib and thalidomide consolidation in transplantation-eligible and transplantation-ineligible patients; thalidomide or lenalidomide maintenance therapy in non-transplantation-eligible patients.
Correspondence
Philip L. McCarthy, Roswell Park Cancer Institute, BMT Program, Department of Medicine, Buffalo, NY 14263; Phone: 716-845-4074; Fax: 716-845-3272; e-mail: philip.mccarthy@roswellpark.org.