Abstract
Transfusion therapy is a key intervention in decreasing morbidity and mortality in patients with sickle cell disease (SCD). Current indications for acute and chronic transfusion therapy have significantly increased the number of RBC units transfused to patients with SCD worldwide. This review summarizes transfusion management for the treatment or prevention of neurologic and perioperative complications, acute chest syndrome, and acute anemia associated with SCD. Despite the recognized benefits of transfusion therapy, it is not without the risks of iron overload, alloimmunization, and delayed hemolytic transfusion reactions. Transfusional iron overload management includes automated RBC exchange, noninvasive imaging to monitor iron burden, and iron chelation with parenteral or oral agents. Although limited and extended RBC antigen matching reduces antibody formation, the prevalence of RBC alloimmunization in patients with SCD remains high. Recent studies demonstrate that RH genetic diversity in patients with SCD contributes to Rh alloimmunization, suggesting that even more refined RBC matching strategies are needed. Advances in molecular blood group typing offer new opportunities to improve RBC matching of donors and recipients and can be of particular benefit to patients with SCD.
Introduction
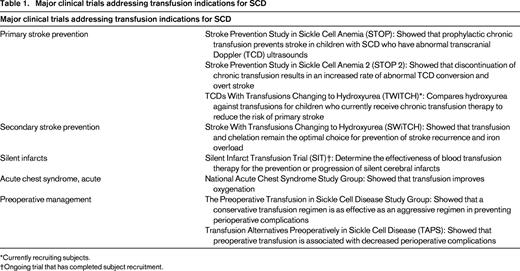
RBC transfusions remain a cornerstone treatment for acute and chronic complications in sickle cell disease (SCD). Approximately 90% of adults with SCD will have received at least one RBC transfusion. Observational studies and randomized clinical trials have demonstrated that RBC transfusions can alleviate or prevent many complications of SCD (Table 1). However, many accepted indications are based primarily on expert consensus, particularly when clinical trials are lacking.1 Overall, the use of transfusion therapy for SCD is increasing due to expanded indications, increased availability of erythrocytapheresis, and oral chelators to prevent transfusional iron overload. The benefits of transfusion therapy must be balanced with the inherent risks. Obligatory iron loading is most problematic for those receiving chronic transfusions and is managed with chelation therapy to prevent hepatic, cardiac, and endocrinologic complications. Alloimmunization remains a major complication associated with RBC transfusions in patients with SCD and can be associated with delayed transfusion reactions with (DHTRs) and without (DTRs) overt hemolysis. Allo- and auto-antibodies increase the complexity of compatibility testing and may delay or prevent the receipt of compatible blood. This review summarizes the main indications for RBC therapy for SCD, focusing specifically on the immunohematological complications and current and emerging strategies to reduce RBC alloimmunization. Transfusion-associated iron overload is the subject of an accompanying chapter.
Indications for RBC transfusion in patients with SCD
Stroke
Stroke is a major cause of long-term morbidity in individuals with SCD, affecting approximately 10% of patients before the age of 20 years without preventative therapy. Acute infarctive stroke is managed with RBC transfusion to reduce the percent hemoglobin S level to below 30% to prevent progression of cerebral ischemia. The optimal transfusion method to provide the best neurologic outcome is not clear. One multi-institutional retrospective study found that exchange transfusion was associated with a lower risk of subsequent stroke compared with simple transfusion at the time of stroke presentation.2 Recurrent stroke occurred in 8 of 14 (57%) patients initially managed with simple transfusions and in 8 of 38 (21%) individuals treated with RBC exchange transfusion. It is possible that initial treatment with exchange transfusion may limit the extent of acute cerebral ischemic injury, thereby decreasing the risk of subsequent stroke, but no study has addressed this question directly. In practice, erythrocytapheresis is the most common method of blood transfusion used for initial treatment of a first stroke.2
Subsequent chronic transfusion therapy helps prevent secondary stroke. Although a controlled clinical trial is lacking, standard care for secondary overt stroke prevention in patients with SCD includes chronic RBC transfusions. Stroke recurs in ∼ 60% of patients without chronic RBC therapy3 and in ∼ 20% of patients despite chronic transfusions and maintaining a hemoglobin S percentage of less than 30%.4,5 Indefinite transfusion therapy is recommended as discontinuation after short-term or long-term prophylactic transfusions has led to recurrent stroke, even with transition to hydroxyurea.6 Patients without a temporally related medical event with their first stroke appear to be at highest risk for recurrent stroke despite maintaining maximum hemoglobin S levels less than 50% or even 30%.4 More recent studies also demonstrate that patients receiving chronic transfusion for secondary stroke prevention are still at risk for silent cerebral infarcts5,7 and cerebral vasculopathy progression.5,8,9 An association between worsening vasculopathy shown by magnetic resonance angiography and progressive overt and silent infarcts on magnetic resonance imaging has been found, suggesting that more aggressive magnetic resonance imaging and therapy may be indicated for patients with stroke and high-risk features.5
A recent randomized phase 3 trial, Stroke With Transfusions Changing to Hydroxyurea (SWiTCH), addressed transition to hydroxyurea therapy for patients with a history of stroke and iron overload.6 Compared with the standard treatment arm (transfusions with iron chelation), there was no difference in liver iron content for patients treated with hydroxyurea and phlebotomy. Subsequently, the trial was closed due to statistical futility on the composite end point of resolution of iron overload and prevention of stroke. At the time of study closure, no strokes were documented in patients receiving transfusions with chelation, but 7 patients (10%) receiving hydroxyurea and phlebotomy had experienced a new stroke.6 Based on these results, transfusion and chelation remain the optimal choice for managing children with SCD, stroke, and iron overload.
Chronic transfusions also prevents initial stroke in high-risk patients identified by transcranial Doppler (TCD) ultrasound. The Stroke Prevention Study in Sickle Cell Disease (STOP) demonstrated a 92% stroke risk reduction among 63 of 130 children with abnormal TCD results.10 Rates of stroke have declined significantly since routine TCD screening and primary prophylactic transfusion therapy for at-risk patients has been universally adopted.11-13 The subsequent STOP 2 trial supports the use of chronic transfusion indefinitely because discontinuation resulted in an increased rate of abnormal TCD conversion and overt stroke.14 Discontinuing transfusions on the STOP 2 trial was also associated with a higher occurrence of silent cerebral infarcts, documented in 3 of 37 patients (8.1%) in the continued-transfusion group compared with 11 of 40 (27.5%) in the transfusion-halted group.15
Two ongoing randomized interventional trials aim to address current dilemmas regarding the use of chronic RBC transfusions to prevent neurologic complications in children with SCD. Comparison of hydroxyurea and transfusion therapy for children with abnormally elevated TCD velocities but no primary stroke is currently ongoing in the TCD with Transfusions Changing to Hydroxyurea (TWiTCH) trial (www.ClinicalTrials.gov identifier NCT01425307). The rationale is based on previous studies demonstrating that hydroxyurea can lower TCD velocities in patients with SCD.16,17 The multi-institutional Silent Infarct Transfusion (SIT) Trial (www.clinicaltrials.gov identifier NCT00072761) has completed subject accrual and will assess the effect of chronic transfusion therapy in preventing progression of silent infarcts in patients with these lesions at baseline and with normal TCD velocities.18 Future studies are needed to determine whether patients with magnetic resonance angiography–defined vasculopathy but normal TCD velocities would benefit from RBC transfusions to prevent vasculopathy progression and/or silent or overt strokes. Young children may also benefit from chronic transfusion therapy to prevent vasculopathy and stroke, because baseline data from the BABY HUG trial identified no patients at a mean age of 12.6 months with abnormal TCD velocities (although norms for this age group have yet to be clearly established).19
Acute chest syndrome
Acute chest syndrome (ACS) describes a new pulmonary infiltrate with respiratory findings such as cough, dyspnea, or new onset hypoxia in a patient with SCD. ACS is the leading cause of death and the second most common cause of hospitalization among patients with SCD. The management of ACS is primarily supportive and includes respiratory therapy, antibiotics, and, often, RBC transfusion. There have been no randomized controlled trials comparing either simple or exchange transfusion versus no transfusion in patients with SCD and ACS.20 However, transfusion therapy improves oxygenation within 12 to 24 hours of RBC administration21 and, in a large epidemiologic study of ACS, management with transfusion was associated with a shorter duration of hospitalization.22 In this same study, nearly three-quarters of the patients received transfusions at the discretion of the treating physician,22 demonstrating its widely accepted use. One small study has shown that simple transfusion is equally effective for the transfusion management of ACS as exchange transfusion.23 Exchange transfusion is typically reserved for patients who are not sufficiently anemic to accommodate a simple transfusion or those with progressive respiratory decline or persistent hypoxia despite simple transfusion.
Because ACS is not the presenting diagnosis in up to half of cases of SCD,22,24 identifying risk factors for preemptive therapy with transfusion is desirable. One small prospective study demonstrated that RBC transfusion provided to patients hospitalized for pain who had elevated secretory phospholipase A2 levels helped to prevent ACS.25 However, a subsequent clinical trial within the SCD Clinical Research Network designed to confirm these findings was closed due to inadequate subject accrual and poor predictive performance of secretory phospholipase A2.
A prospective, randomized trial comparing the efficacy of chronic transfusions versus hydroxyurea to prevent recurrent ACS has not been conducted. Hydroxyurea use is associated with lower rates of ACS26 and is indicated for prevention of recurrent ACS.27 Chronic transfusion therapy is sometimes offered, particularly to those who experienced severe ACS requiring mechanical ventilation or erythrocytapheresis after failure to respond to simple transfusions. A dramatic reduction in hospitalization for ACS (and pain) was observed in children undergoing chronic transfusion for primary stroke prevention compared with the observed group.28 However, whereas chronic transfusion therapy reduces the incidence of ACS events among patients with recurrent ACS, it may not necessarily reduce the severity of episodes.29
Acute exacerbation of anemia
Simple transfusion is indicated for acute exacerbation of anemia occurring with acute splenic sequestration and transient RBC aplasia. Acute splenic sequestration is typically accompanied by a precipitous decrease in hemoglobin level and the potential for hypovolemic shock. The immediate treatment is directed toward correction of hypovolemia with RBC transfusion. Because RBCs sequestered in the spleen are remobilized, patients should be transfused cautiously to prevent hyperviscosity after splenic sequestration resolves. Aliquots of 5 mL/kg may be administered, along with close monitoring of the spleen size, hemoglobin level, and cardiovascular status of the child. In cases of severe sequestration and anemia with hypovolemic shock, initial transfusion with 10 mL/kg RBCs is appropriate. In a retrospective multicenter study of 190 patients with SCD of genotypes SS or Sβ0, 67% of infants with splenic sequestration had one or more recurrent episodes.30 Watchful waiting without prophylactic blood transfusion or splenectomy was used in 54% of patients in this cohort, of which 59% experienced no further episodes. Chronic RBC transfusion to prevent recurrent splenic sequestration has not been prospectively studied and remains controversial; in practice, it is sometimes used to delay splenectomy in very young children, but splenectomy remains the definitive treatment. Severe anemia also occurs with transient aplastic episodes due to temporary suppression of erythropoiesis given the significantly shortened lifespan of RBCs in patients with SCD. In a single institution observational study of Parvovirus B19–induced RBC aplasia, the median nadir hemoglobin was 4.8 gm/dL and 49 of 68 pediatric patients (72%) received a simple RBC transfusion.31 Because the anemia associated with RBC aplasia is subacute, the patient is typically euvolemic and physiologically compensated. Therefore, RBC transfusion should be administered slowly with serial small aliquots to prevent congestive heart failure.
Preoperative transfusion
Perioperative conditions including suboptimal hydration, poor oxygenation, and acidemia can lead to SCD-related complications such as ACS, painful vasoocclusive episodes, and infections. Nearly 2 decades ago, a randomized controlled trial showed that preoperative simple transfusion to achieve a hemoglobin of 10 g/dL is equally effective in preventing postoperative complications compared with aggressive transfusion therapy aimed to decrease the percent hemoglobin S level to < 30% via erythrocytapheresis.32 Only recently, the Transfusion Alternatives Preoperatively in SCD (TAPS) trial compared the outcomes of preoperative transfusion versus no preoperative transfusion in patients with HbSS or Sβ0 thalassemia undergoing low-risk or medium-risk surgery in a prospective, randomized study.33 This study, which was terminated early due to an imbalance of adverse events, demonstrated that preoperative transfusion is associated with decreased perioperative complications in patients with SCD, with 13 of 33 (39%) patients in the no preoperative transfusion group experiencing a clinically important complication compared with 5 of 34 (15%) patients who were transfused preoperatively. However, the TAPS trial was not able to address 2 major questions, what is the best preoperative management of individuals with other subtypes of SCD (HbSC or Sβ+ thalassemia) and what is the optimal management for low-risk procedures, due to the small number of enrolled patients (n = 13) in this category.
Other considerations
Transfusion therapy to prevent chronic organ damage (osteonecrosis, retinopathy, renal failure) from vasoocclusion, chronic hemolysis, and blood viscosity has not been studied. Pulmonary hypertension affects up to 30% of adults with SCD and strongly predicts morbidity,34 but there is no proven treatment. Chronic RBC transfusions and long-term anticoagulation have been suggested but require investigation. Pregnant women with SCD have increased maternal and fetal mortality and morbidity. RBC transfusion is indicated for the treatment of acute complications during pregnancy, but there are insufficient data currently to recommend its use prophylactically.35 There have also been no clinical trials to determine the effectiveness of transfusion therapy for acute management or prevention of vasoocclusive painful episodes, priapism, or prevention of other end organ damage due to SCD.
Complications of transfusion therapy
RBC transfusions pose inherent risks to all recipients, including infection, iron overload, allergic reactions, alloimmunization, and acute or delayed hemolytic transfusion reactions (DHTRs). The remainder of this review focuses on alloimmunization in patients with SCD, as well as current and future strategies to minimize risk.
RBC alloimmunization in patients with SCD
Alloimmunization to RBC antigens is a major complication associated with RBC transfusions in patients with SCD. Alloantibodies and autoantibodies complicate RBC cross-matching, delay provision of transfusions, and increase the labor and cost of providing compatible RBC units. The high rate of alloimmunization in patients with SCD is multifactorial. The discordance of blood group antigen expression on donor and patient RBCs is likely the most important contributing factor, but transfusion burden, degree of antigen matching, and age at first transfusion are also major determinants. More recently, insights into the genetic heterogeneity of the Rh blood group system, particularly in individuals of African descent, provides a molecular basis for many complex Rh specificities seen in patients with SCD, but also provides new challenges to RBC matching for this population.
Prevalence of alloimmunization
In the United States, the incidence of alloimmunization in the general population has been estimated to be approximately 0.5% to 1.5%. In comparison, the incidence of alloimmunization in patients with SCD ranges from 18% to 76% with ABO and D matching alone; 5% to 14.5% with limited phenotype matching for C, E, and K antigens; and 7% for extended minor RBC antigen-matching beyond C, E, and K (for review, see Chou et al,36 Lasalle-Williams et al,37 O'Suoji et al38 ). Alloantibodies to the Rh (primarily C and E) and Kell (typically K) systems comprise more than 2/3 of the RBC antibodies detected in patients with SCD. Uncommon specificities in these systems also affect patients with SCD and include antibodies to the V/VS, hrB, Goa, and Jsa antigens. In addition, clinically significant alloantibodies in the Kidd (Jkb>Jka), Duffy (Fya>Fyb), and MNS (primarily S) systems also occur. Many patients with SCD have multiple alloantibodies that can complicate pretransfusion serologic evaluations and delay finding compatible RBCs. In fact, the American Rare Donor Program receives the most requests for rare units of RBCs for alloimmunized patients with SCD.
Despite prophylactic C, E, and K matching, many patients with SCD continue to form antibodies with common Rh specificities (D, C, E, c, e). In a recent single-institution study of pediatric patients with SCD for whom limited RBC antigen-matching for C, E, K is practiced, 16 of 180 transfused patients formed 9 anti-C, 7 anti-E, and 5 anti-K antibodies.38 The majority of cases were ascribed to transfusion at outside institutions that may not provide C-, E-, and K-matched RBCs, but 5 antibodies against Rh antigens occurred in patients who had Rh variants revealed by RH genotyping. We also recently reported a high rate of Rh alloimmunization in 182 transfused patients with SCD despite C-, E-, and K-matched RBCs primarily from African-American donors.39 More than two-thirds of antibodies were directed against the Rh system; 45% of chronic and 12% of episodically transfused patients were Rh immunized. High-resolution RH genotyping revealed that ∼ 30% of Rh antibodies occurred in patients homozygous for RH variant alleles. We found tremendous RH diversity in this population, with the majority of patients carrying at least one variant RH allele and 43% with both variant RHD and RHCE. However, not all Rh antibodies in this cohort could be explained by patient homozygosity for altered alleles at the corresponding RH loci. Therefore, we hypothesize that Rh antibodies are also formed in response to African-American donor RBCs that express variant Rh antigens. Future studies will determine whether RBCs from African-American donors stimulate these complex Rh specificities. In summary, despite limited RBC antigen matching, alloimmunization still occurs due to Rh variants in patients and donors that are not detected by routine serology and transfusion at institutions where prospective C-, E-, and K-antigen matching is not performed.
DTRs
DHTRs, characterized by fever, pain, and signs of hemolysis (dark urine, jaundice, pallor) occurring a few days to 2 weeks after a transfusion, represent a potentially life-threatening complication of RBC therapy. The incidence of DHTRs in patients with SCD is not well established, but small case series estimate that ∼ 5% of transfused patients with SCD will experience a DHTR.40 It is not uncommon for DHTRs to occur in patients with SCD in the absence of a detectable antibody or a positive DAT.41,42 DTRs can also occur without overt clinical signs of hemolysis39 and posttransfusion screening tests may be negative.42 Hyperhemolysis syndrome is a severe complication described in patients with SCD in which the patient's hemoglobin drops lower than the pretransfusion hemoglobin, suggesting hemolysis of autologous RBCs in addition to donor RBCs. Possible mechanisms of hyperhemolysis syndrome include bystander hemolysis, suppression of erythropoiesis, and RBCs being destroyed by activated macrophages.43
The management of DHTRs remains controversial, particularly when antibodies cannot be detected and with hyperhemolysis syndrome. If the antibody specificity is determined and the patient requires transfusion, the administration of RBCs lacking the antigen is usually safe. In cases of hyperhemolysis syndrome, further transfusion may exacerbate ongoing hemolysis, so individual management is dependent on the severity of anemia and the rapidity of hemolysis.42,43 In less severe cases, transfusion may be avoided with close monitoring and the patient should be treated with corticosteroids with or without IVIg. In cases of severe hemolysis and hyperhemolysis, the patient will likely require transfusion and corticosteroids and IVIg should be used in conjunction.42,43 Rituximab and erythropoietin have both been used for the management of DHTRs and hyperhemolysis, but larger studies are needed to determine their role. DHTRs and DTRs may be underestimated in patients with SCD, so future prospective studies are needed to better understand the pathophysiology, risk factors, and optimal management of this serious complication of RBC therapy.
RH diversity contributes to alloimmunization
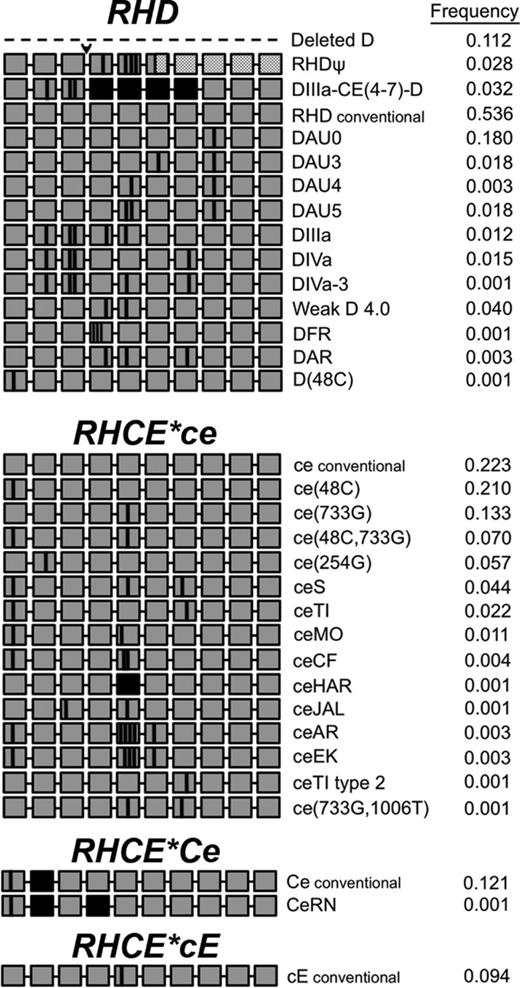
The RHD and RHCE genes lie in close proximity and encode the D antigen and the CE antigens in various combinations (ce, cE, Ce, or CE), respectively. The conventional RH genes are found in all population groups, although with different frequencies. Commercial antibody reagents detect expression of the 5 principal Rh antigens: D, C, c, E, and e. In the past decade, the genetic diversity of the RH loci has been revealed, with more than 200 RHD and 80 RHCE alleles reported. Variant RHD and RHCE alleles encode Rh proteins with amino acid changes that cannot be distinguished with common serologic reagents, but can be recognized by the immune system as foreign (for review, see Chou et al44 ). Although RHD and RHCE variants are found in < 1% to 2% of Europeans, the frequency in individuals of African descent appears to be much higher. Specific Rh variants are more frequent in individuals of African descent and therefore may often be found in patients with SCD of African ancestry. These RBCs may lack common Rh antigen epitopes (eg, hrB or hrs) or carry new epitopes (eg, V, VS, Goa). Figure 1 illustrates the RH allele prevalence and variants identified in a cohort of 320 patients with SCD followed at our institution.
RHD and RHCE diversity in 320 patients with SCD.RH alleles identified in patients with SCD. Each grey box represents 1 of 10 exons in the RH genes. Black boxes represent exon exchange between RHD and RHCE. Vertical black lines indicate positions encoding amino acid substitutions in the protein. Dashed line indicates RHD gene deletion. Arrowhead indicates the 37-bp duplication. Hatched boxes represent exons encoding a frameshift and untranslated region of the inactive RHD pseudogene.
RHD and RHCE diversity in 320 patients with SCD.RH alleles identified in patients with SCD. Each grey box represents 1 of 10 exons in the RH genes. Black boxes represent exon exchange between RHD and RHCE. Vertical black lines indicate positions encoding amino acid substitutions in the protein. Dashed line indicates RHD gene deletion. Arrowhead indicates the 37-bp duplication. Hatched boxes represent exons encoding a frameshift and untranslated region of the inactive RHD pseudogene.
Variations in RHD
In most populations, the D-negative (D−) phenotype of the Rh blood group system results almost invariably from deletion of the entire RHD gene. Rare exceptions to this molecular basis of the D− phenotype have been described. However, in a cohort of 82 D− South-African donors, a minority had complete deletion of RHD. Sixty-six percent had an RHD pseudogene (RHDψ) that contains a 37-bp insertion that results in a premature stop codon, 15% had a DIIIa-CE(4-7)-D hybrid gene characterized by C-antigen expression but no D, and only 18% had deletion of the entire RHD gene.45 Of 320 African-American patients with SCD, 16 phenotyped D−, of which 12 were homozygous for RHD gene deletion, 2 were homozygous RHDψ, and 2 were heterozygous for the gene deletion (1 had RHDψ and 1 had DIIIa-CE(4-7)-D, in trans). Therefore, in different African subpopulations, RH allele frequency may be unique.
Most individuals who are D+ also have identical RHD sequences, but ∼2% have variant RH alleles. The altered RhD proteins can cause reduced expression of antigen, termed weak D,46 and/or result in D antigens lacking certain epitopes, termed partial D. Individuals with weak D expression are not typically at risk of anti-D production. In contrast, patients with partial D can develop anti-D when stimulated by conventional D+ RBCs and can experience delayed transfusion reactions with and without overt hemolysis.39,47 In our cohort of 320 patients, 49% of D+ patients had conventional RHD only, 30% had at least one variant and one conventional RHD, and 21% had only variant RHD alleles (either homozygous or compound heterozygous; Table 1). Of the individuals with only variant RHD alleles, 71% had 1 or 2 DAU0 alleles, which has a similarly high frequency in African-American donors.
A frequent variant RHD allele in individuals of African descent is the hybrid RHD*DIIIa-CE(4-7)-D gene in which several RHCE exons have replaced the corresponding region of RHD.45 This gene does not encode the D antigen, but rather a partial C antigen. It is often inherited with an RHCE allele designated ceS, which encodes altered e antigen and a V−VS+ phenotype. The hybrid RHD*DIIIa-CE(4-7)-D gene linked to RHCE*ceS is referred to as the r′S haplotype. RBCs with the r′S haplotype type strongly C+ with monoclonal reagents and the presence of the altered C goes undetected. These patients not infrequently make alloantibodies with C-like and e-like specificities that can appear to be autoantibodies.48 Their RBCs also typically lack the high-prevalence hrB antigen. Anti-hrB can be clinically significant and finding compatible blood can be very difficult.49,50 Of 320 patients with SCD, we identified 24 (7.5%) who inherited the r′S haplotype; 6 (25%) made anti-C and 3 (12.5%) formed anti-e.
Variations of RHCE
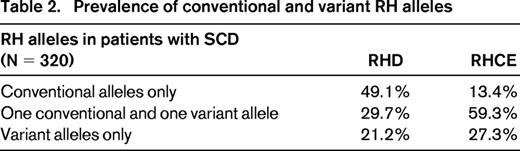
RHCE encodes both C/c and E/e antigens on a single protein in various combinations (ce, cE, Ce, or CE). Variant RHCE is relatively common in African ethnic groups and results in quantitative and qualitative changes, with altered e encountered most frequently. The RBCs type as e+, but individuals homozygous for these alleles make alloantibodies with e-like specificities that can appear to be autoantibodies. Certain variants are associated with RBCs that lack the high-prevalence hrS antigen, so patients can form anti-hrS, which has been associated with clinically significant DTRs.51 In our patient cohort, 13% of patients had only conventional RHCE alleles, 60% had at least 1 variant RHCE and 1 conventional RHCE, and 27% had only variant RHCE alleles (either homozygous or compound heterozygous; Table 2). Of those with only variant RHCE alleles, 62% had at least one RHCE*ce(48C) allele, which is a common allele among individuals of African descent. Altered RHCE*ce is often inherited with partial RHD alleles, such as DIII, DAU, and DAR. The inheritance of partial D alleles with variant RHCE*ce in patients with SCD puts them at risk for the production of antibodies to both RhCE and RhD proteins, a potentially serious complication.
Thus, many patients with SCD who develop anti-D, anti-C, and/or anti-e despite their own RBCs testing positive for those antigens using routine serologic reagents, may represent Rh alloantibody rather than autoantibody formation. These can be clinically significant and future transfusions should be with antigen-negative RBCs. This poses significant challenges to transfusion services in providing compatible RBCs to these patients because care must be taken to avoid the development of additional alloantibodies.
Strategies to prevent alloimmunization
RBC antigen-matching strategies
Although single-institution and prospective multi-institutional studies have shown that limited C-, E-, and K-phenotype–matched RBCs significantly reduce the incidence of alloantibody production in SCD,37,38,52 there remains no universal standard of care. More than 90% of institutions with Comprehensive Sickle Cell Centers provide RBCs that are prophylactically matched for C, E, and K to patients with SCD,53 but only one-third of transfusion services nationally have such a policy.54 A minority of institutions have protocols for extended RBC matching beyond C, E, and K, most commonly for the Jka, Jkb, Fya, Fyb, and S antigens, which can further minimize alloimmunization risk. Some transfusion services provide extended matched RBCs after a patient with SCD has formed one alloantibody, recognizing these patients as “immune responders.”55 The recruitment of dedicated donors and limiting the number of donors for a given patient has also been a strategy used for patients with SCD.56
Even when donors and recipients are ethnically alike and share more similar RBC antigen profiles, relatively high rates of alloimmunization persist. In a study of Ugandan patients with SCD, 6.1% were alloimmunized despite low transfusion exposure and ethnic similarity between donors and recipients.57 Our recent study evaluating the effect of C-, E-, and K-matched RBCs combined with transfusion of African-American donor units demonstrated a high rate of alloimmunization despite these strategies. RH variation in patients and donors both contribute to complex Rh alloimmunization and this evidence suggests an emerging role for DNA-based testing to improve RBC matching.38,39,48
Molecular blood group antigen typing
Most allelic blood group antigens result from single nucleotide polymorphisms and the majority of those responsible for the polymorphic antigens associated with clinically significant antibodies have been identified. High-throughput platforms to genotype RBC antigens are now readily accessible and can detect conventional RBC antigens from more than a dozen blood group systems, including Rh, Kell, Duffy, Kidd, Diego, Dombrock, Colton, Lutheran, Scianna, and MNS.58 To provide antigen-negative RBCs to patients with SCD with the type commonly needed (RBCs lacking C, E, K, Fya, and Jkb), blood from African-American donors must be screened because this phenotype is considerably more prevalent in that population than among whites. Two feasibility studies have demonstrated that molecular genotyping of donors and patients with SCD facilitates extended RBC matching of conventional blood group antigens.59,60 The application of molecular technologies for patients and donors will undoubtedly affect rates of alloimmunization in SCD, as well as other chronically transfused populations.
High-resolution RH genotyping is currently limited to reference molecular immunohematology laboratories. However, commercial PCR-based technology has been developed and used to detect the majority of common Rh variants on high-throughput genotyping platforms.39 RH genetic testing detects altered RHD and RHCE alleles and aids in the identification of individuals at risk for producing Rh antibodies. We currently advocate RH genotyping for all patients with SCD given the high prevalence of Rh variants found in this population. For patients with select predicted partial antigen expression, we provide antigen-negative RBCs. To prevent anti-C formation, patients with the hybrid RHD*DIIIa-CE(4-7)-D gene that encodes a partial C antigen should prophylactically receive C− RBCs if they do not carry an RHCE allele encoding conventional C protein. Providing D− RBCs for patients predicted to exclusively express partial D antigen is currently performed on an individual case basis, with consideration to their extended RBC antigen profile, their alloimmunization history and specificities, and the predicted availability of appropriate donor RBCs.
High-throughput RBC genotyping for conventional blood group antigens and for RH variants have the potential to dramatically increase inventories of antigen-negative blood, which would significantly affect transfusion therapy for patients with SCD. Chronically transfused patients would undoubtedly benefit from extended antigen-matched RBCs to minimize alloimmunization. Prevention of complex Rh alloantibodies may be feasible by providing antigen-negative RBCs to patients with unusual Rh phenotypes, such as those with e variants that result in RBCs lacking the high-prevalence hrS and hrB antigens. Patients with common partial D variants could receive RHD genotype-matched RBCs rather than D− units, which are also limited in supply. Moreover, donor RBC genotyping could expand the inventory of RBCs for patients who have already formed high-prevalence antigens, reduce labor- and time-intensive procedures to finding compatible blood for patients with warm autoantibodies, and provide compatible blood to patients with antibodies for which there is no antiserum (V/VS, Goa, Jsa, Doa, Dob), thus improving transfusion safety. Future studies are needed to address whether RH genotyped-matched RBCs for patients with SCD can prevent the Rh alloimmunization observed despite provision of Rh-matched transfusions.
Conclusion
RBC transfusions remain an essential component of the medical management of patients with SCD. Transfusions decrease the morbidity of acute complications, reduce the recurrence of SCD-associated complications, and prevent neurologic events in patients with high-risk features. As the indications for transfusion expand for this population, the incidence of RBC alloimmunization also increases. The management of alloimmunization in SCD has been the subject of much debate and currently there is no single approach. Universal approaches to minimizing RBC alloimmunization are needed to reduce complications and improve RBC therapy. The recent advances in blood group genotyping can be exploited to facilitate the identification of antigen-matched RBCs and improve transfusion support of patients with SCD. Automated DNA extraction and the ability to test patients and donors on high-throughput platforms, combined with database-driven RBC matching, should make this possible in the near future.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Stella T. Chou, MD, Assistant Professor of Pediatrics, Division of Hematology, The Children's Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania, 3615 Civic Center Blvd, ARC 316D, Philadelphia, PA 19104; Phone: 215-590-0947; Fax: 215-590-4834; e-mail: chous@email.chop.edu.