Abstract
Applying the principles of evidence-based medicine to febrile neutropenia (FN) results in a more limited set of practices than expected. Hundreds of studies over the last 4 decades have produced evidence to support the following: (1) risk stratification allows the identification of a subset of patients who may be safely managed as outpatients given the right health care environment; (2) antibacterial prophylaxis for high-risk patients who remain neutropenic for ≥ 7 days prevents infections and decreases mortality; (3) the empirical management of febrile neutropenia with a single antipseudomonal beta-lactam results in the same outcome and less toxicity than combination therapy using aminoglycosides; (4) vancomycin should not be used routinely empirically either as part of the initial regimen or for persistent fever, but rather should be added when a pathogen that requires its use is isolated; (5) empirical antifungal therapy should be added after 4 days of persistent fever in patients at high risk for invasive fungal infection (IFI); the details of the characterization as high risk and the choice of agent remain debatable; and (6) preemptive antifungal therapy in which the initiation of antifungals is postponed and triggered by the presence, in addition to fever, of other clinical findings, computed tomography (CT) results, and serological tests for fungal infection is an acceptable strategy in a subset of patients. Many practical management questions remain unaddressed.
Evidence-based medicine
Practicing evidenced-based medicine means “integrating individual clinical expertise with the best available external clinical evidence from systematic research.”1 This definition acknowledges that anyone's personal experience is limited but valuable, and then assumes that systematic research has produced evidence applicable to the particular case. The key point is the qualifier “best,” meaning that the evidence has to be appraised, which is not easy. Some helpful online resources that focus on evidence-based medicine include http://www.cebm.net, http://plus.mcmaster.ca/EvidenceUpdates/Default.aspx, and http://acpjc.acponline.org/.
The “right” hierarchy of evidence is a matter of academic debate, although the principles are agreed upon: a properly conducted randomized controlled trial (RCT) is usually better evidence than an observational study, which is generally better than a case series, which beats a case report. A systematic review of all RCTs usually is preferable over a single trial. At the bottom of the ladder is “mechanism-based reasoning” (very frequently used during ward rounds when there is nothing better). An example of how different levels of evidence may support different conclusions regarding the use of vancomycin in neutropenic patients is presented in Table 1. This example uses the ranking proposed by the Oxford-based Centre for Evidence-based Medicine (CEBM).2,3
Example of how recommendations vary depending on the levels of evidence: vancomycin in FN
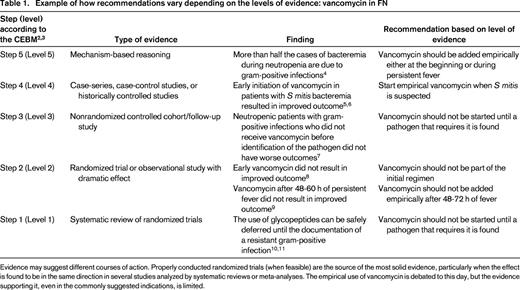
Evidence may suggest different courses of action. Properly conducted randomized trials (when feasible) are the source of the most solid evidence, particularly when the effect is found to be in the same direction in several studies analyzed by systematic reviews or meta-analyses. The empirical use of vancomycin is debated to this day, but the evidence supporting it, even in the commonly suggested indications, is limited.
When confronted with a clinical decision, instead of personally sieving through the evidence, one may look up guidelines offered by professional societies or ask for the opinion of an expert. EBM's most important concept is that any recommendation must be supported by evidence and that the quality of the evidence must be made explicit.
Professional organizations have indeed embraced some form of assessment of the evidence in their published guidelines. Unfortunately, different organizations keep using different grading systems, creating a sometimes confusing amalgam of letters and numbers to rank the strength of a given recommendation and the quality of the evidence on which the recommendation is based (Table 2). This may make the interpretation of guidelines more cumbersome than in the past. For example, the very first Infectious Diseases Society of America (IDSA) guideline for the management of febrile neutropenia (FN) had a simple “star rating scheme similar to that used for theatrical productions,”12 which was easier to follow than the current “A–D” and “I–III.”13
Evidence grading used on selected recent guidelines for fever and neutropenia

IPF NGP indicates International Pediatric Fever and Neutropenia Guideline Panel.
This chapter provides a review and comments on a selection of the evidence-based guidelines for fever and neutropenia published recently. The terminology used by the guidelines issued by the European Society for Medical Oncology (ESMO),14 the IDSA,15 the National Comprehensive Cancer Network (NCCN)16 and the American Society of Clinical Oncology (ASCO)17 are presented on Table 2.
ASCO has also endorsed the pediatric guidelines proposed by the International Pediatric Fever and Neutropenia Guideline Panel for the Management of Fever and Neutropenia in Children with Cancer and/or Undergoing Hematopoietic Stem Cell Transplantation.20 These are notable for being the first fever and neutropenia guidelines that explicitly use the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) approach to qualify the strength of the recommendation and the quality of the evidence. The GRADE framework (http://www.gradeworkinggroup.org/), recently adopted also by the IDSA, is an attempt to develop a universal system to formulate and grade evidence-based practice guidelines. It explicitly separates “strength of recommendation” from “quality of the evidence” and proposes unambiguous definitions. Recommendations can only be “strong” or “weak” (based on whether the desirable effects of the recommendation outweigh the undesirable effects)21 and the quality of evidence can only have 4 levels: high, moderate, low, and very low (based on how likely it is that future research will change the estimate of the effect or even the direction of the effect).22 Once the basic concept is accepted, the classification is easy to follow and logical: an intervention that is potentially lifesaving may get a strong recommendation even when the evidence supporting it is of low quality (eg, empirical addition of antifungal therapy in children with persistent FN20 ) and, conversely, very-high-quality evidence may generate only a weak recommendation if the undesirable consequences have not been fully explored (eg, fluoroquinolone prophylaxis in afebrile patients who are expected to remain neutropenic for ≥ 7 days, supported by meta-analyses but rejected by the Australian guidelines23 ). Critical appraisal of the evidence is essential, but leaves room for subjectivity. Randomized trials begin as high-quality evidence and observational studies as low-quality evidence, but the former may be downgraded (eg, because of lack of blinding or variability in results) and the latter upgraded (eg, because of a very large magnitude of effect).
Evidence-based guidelines for FN
In the following sections, I will summarize recommendations regarding prophylaxis of fever during neutropenia, risk stratification, and the 4 distinct fever syndromes characterized by Bow in his review.24 A uniform structure will be used: definition, background, recommendation from the guidelines, and highlights/limitations of the evidence.
Some limitations of the evidence generated in FN studies should be mentioned. First, fever is not a clinicopathological entity. Fever is used as a surrogate marker for infection, but in fact is a diagnostic test of unclear sensitivity and specificity. An undetermined fraction of patients with fever (who were included in the trial) do not have infection and another fraction of patients without fever (who were excluded from the trial) do in fact have an infection that could benefit from treatment with the antimicrobial agent. This means that the population of interest and the effect of the intervention may be diluted out due to misclassification. Second, for a wide array of daily clinical decisions in the management of neutropenic patients, there is only very low-quality evidence (or no evidence at all), usually in the form of case reports and case series, and the guidelines remain silent (Table 3).
Examples of clinical scenarios in FN for which evidence-based recommendations are not available

All patients were seen by the author at the NIH Clinical Center. Patient 1 was downgraded to ceftriaxone; patient 2 was treated with piperacillin-tazobactam and defervesced uneventfully; patient 3 was started on voriconazole, but the bronchoalveolar lavage showed Cunninghamella and he was successfully treated for mucormycosis with liposomal amphotericin B and surgical resection; patient 4 had Enterococcus faecalis bacteremia; patient 5 had CMV pneumonitis.
AML indicates acute myelogenous leukemia; VRE, vancomycin-resistant enterococcus; ANC, absolute neutrophil count; and ALL, acute lymphocytic leukemia.
There are hundreds of trials comparing antibiotics for the first episode of fever, but one of the most common scenarios, recrudescent fever (a second episode of fever after the first one resolved with antimicrobial treatment), has barely been addressed and it is not easy to envision a RCT that could do it properly. The Pediatric Fever and Neutropenia Guideline Panel has presented a list of “research gaps” in FN in their Guidelines.20
Prevention of fever during neutropenia using antimicrobial agents
Definition
Antimicrobial agents used in afebrile neutropenic patients with the aim of decreasing infections and death. Fever may be the primary end point of some of the studies, but it is worth noting that the use of anti-infective drugs must logically be aimed at preventing infection.
Background
The use of antimicrobials to prevent infections has always been controversial. As a general rule, prophylaxis tends to work (ie, the incidence of the infection of interest decreases). The controversy derives from the assessment of the unintended (and frequently unmeasured) consequences of prophylaxis and the analysis of the cost/benefit ratio of the intervention for the individual patient, the hospital, and the community.
Recommendations from the guidelines
Antibacterial prophylaxis.
ESMO,14 IDSA,13 ASCO,17 and NCCN16 recommend antibacterial prophylaxis with a fluoroquinolone for patients who are going to be neutropenic for ≥ 7 days. The Australian guidelines recommend avoiding antibacterial prophylaxis in general and consider it only for stem cell transplantation patients and palliative patients with BM failure.23
Antifungal prophylaxis.
Antifungal prophylaxis recommendations are particularly complex in all guidelines because they require an assessment of the risk of Candida versus mold (mainly Aspergillus) infection. For outpatients, ASCO recommends antifungal prophylaxis with a triazole in outpatients who are expected to be neutropenic for ≥ 7 days.17 The IDSA recommends fluconazole prophylaxis for patients at risk for Candida and advises considering posaconazole for selected patients undergoing intensive chemotherapy for acute leukemia or myelodysplastic syndrome13 based on a single RCT that showed improvement in overall survival in this group.25 The NCCN divides patients in low, intermediate, and high risk of fungal infections and provides detailed examples of each category with the recommendation to “consider” a variety of antifungal prophylaxis agents with different degrees of consensus.16 The Australian guidelines provide very specific pathogen-specific recommendations.26
Highlights and limitations of the evidence
Antibacterial prophylaxis.
Several meta-analyses support a beneficial effect in fever, documented infections, and overall mortality by administering fluoroquinolone prophylaxis to patients who are neutropenic for ≥ 7 days.27-30 The low quality of many of the studies included in the meta-analyses, the lack of an effect on mortality in any single trial (including the latest and more influential31 ), and the paucity of long-term data on bacterial resistance and patient colonization with resistant pathogens are the arguments for the Australian guidelines to not recommend its routine use.23
Antifungal prophylaxis.
There is convincing evidence that fluconazole reduces the rates of invasive candidiasis32 and death33 in patients at high risk of fungal infection. There is also evidence that antifungal agents with activity against Aspergillus are more effective than fluconazole in reducing the rates of invasive aspergillosis both during neutropenia34-36 and in other high-risk settings37,38 However, there is only one RCT (not blinded) showing that a mold-active drug (posaconazole) improved survival compared with fluconazole or itraconazole.25 The conflict between the biological effect (less aspergillosis) and the clinical outcome effect (improved survival in only one trial) makes it difficult to formulate unambiguous guidelines.
Risk stratification
Definition
Patients who develop neutropenia during chemotherapy for cancer can be categorized as at high risk or low risk of a poor outcome based on a variety of factors.
Background
The mortality of an episode of fever and neutropenia varies widely.39 The risk of complications and poor outcomes has practical implications for management (choice of antibiotics, inpatient vs outpatient setting).
Recommendations from the guidelines
The adult guidelines from Australia,40 ESMO,14 and ASCO17 recommend the use of the Multinational Association for Supportive Care in Cancer (MASCC) index41 to identify patients at low risk of complications who could be treated as outpatients. The NCCN guidelines offer a more detailed discussion of risk of infection (beyond neutropenia), but they also support the use of the MASCC index.16 The pediatric guidelines also support the concept of risk stratification and emphasize the importance of using strategies that have been validated locally.20
Highlights and limitations of the evidence
The most commonly endorsed stratification strategy is the MASCC index, which was designed as a tool to identify adult patients at low risk of complications.41 To obtain a MASCC score, points are allocated and added up. Points are given for burden of illness (no or mild symptoms = 5 points, severe symptoms = 3 points), absence of hypotension (5 points), absence of chronic obstructive pulmonary disease (4 points), solid tumor OR no previous fungal infection (4 points), absence of dehydration (3 points), outpatient status (3 points) and age < 60 years (2 points). The points are added up, and patients with a score of ≥ 21 points (of 26 possible) are considered “low risk” and can be considered for oral therapy. The index has been validated in multiple settings and performs well, although it may function better in solid tumors than in hematologic malignancies.39 Although most guidelines recommend its use, some point out potential limitations and provide specific clinical criteria to supplement the MASCC score and improve its discriminating power. The IDSA guidelines make a distinction between “expert” clinical criteria derived from clinical trials and the MASCC index: patients with neutropenia expected to last ≥ 7 days, those who are clinically unstable or with significant comorbidities, and those with some underlying cancers or high-intensity chemotherapy are all “high risk” and the recommendation is hospitalization and IV antibiotics.13 Similarly, the ASCO guidelines present a list of conditions that makes the outpatients high risk independently of their MASCC score.17 Needless to say, even if the conditions proposed by the guideline developers seem to be perfectly sound, until they are validated prospectively, they must be considered expert opinion.
Fever and neutropenia syndromes24
First episode of fever
Definition.
Fever is a single oral temperature ≥ 38.3°C or sustained temperature ≥ 38°C for 1 hour.13 Slightly different definitions may have been used in different trials over the years. Neutropenia is defined as an absolute neutrophil count of < 500 cells/mm3 or that is expected to decrease to < 500 cells/mm3 during the next 48 hours.13
Background.
Most, if not all, episodes of fever during neutropenia are supposed to be infectious in origin. Infection, however, is documented only in a minority of cases. The percentages are roughly as follows: fever of unknown origin 50%-60%; microbiologically documented infection (frequently bacteremia) 10%-20%; clinically documented infection (eg, typhlitis or cellulitis without any pathogen being isolated) 20%-30%.13 Much higher rates of bacteremia were documented before the current practice of early initiation of empirical antibiotics.42
Recommendations from the guidelines.
For high-risk patients, all of the guidelines recommend starting monotherapy with a beta-lactam with activity against Pseudomonas aeruginosa (piperacillin-tazobactam, imipenem, meropenem, cefepime, ceftazidime) and add the important caveat that some form of combination therapy should be chosen in patients who are clinically unstable and when there is suspicion (or high risk) of infection caused by resistant gram-negative (a second gram-negative agent should be added) or gram-positive bacteria (vancomycn or linezolid should be added). All the guidelines recommend not including vancomycin routinely in the initial regimen and not adding it empirically for persistent fever. The details regarding when to actually use vancomycin are more variable. The IDSA strongly recommends adding vancomycin in cases of hemodynamic instability, pneumonia, clinically evident catheter-related infection, skin and soft tissue infections, severe mucositis when fluoroquinolone prophylaxis has been used and ceftazidime is used empirically, and known colonization with methicillin-resistant Staphylococcus aureus, although the quality of the evidence is low.13
For patients who are considered “low risk” and eligible for outpatient management, the regimen of choice is the combination of fluoroquinolone and amoxicillin-clavulanic acid (or clindamycin for penicillin-allergic patients) as long as no fluoroquinolone prophylaxis was used, the patient tolerates oral medication, and the rate of resistance to fluoroquinolones is less than 20%.17 It is likely that moxifloxacin monotherapy will be supported in future editions of the guidelines.43 When a fluoroquinolone cannot be used, a broad-spectrum beta-lactam active against Pseudomonas and suitable for outpatient use should be used.
Highlights and limitations of the evidence.
The first episode of fever during neutropenia has been studied extensively and recommendations for initial management get the strongest recommendation with the highest quality evidence in all the guidelines (Table 4).
Evidence-based recommendations for FN syndromes
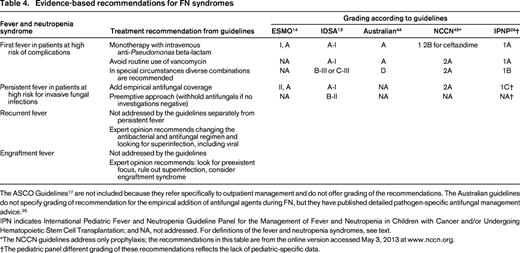
The ASCO Guidelines17 are not included because they refer specifically to outpatient management and do not offer grading of the recommendations. The Australian guidelines do not specify grading of recommendation for the empirical addition of antifungal agents during FN, but they have published detailed pathogen-specific antifungal management advice.26
IPN indicates International Pediatric Fever and Neutropenia Guideline Panel for the Management of Fever and Neutropenia in Children with Cancer and/or Undergoing Hematopoietic Stem Cell Transplantation; and NA, not addressed. For definitions of the fever and neutropenia syndromes, see text.
*The NCCN guidelines address only prophylaxis; the recommendations in this table are from the online version accessed May 3, 2013 at www.nccn.org.
†The pediatric panel different grading of these recommendations reflects the lack of pediatric-specific data.
Two systematic reviews and a meta-analysis support the recommendation of the intravenous antipseudomonal beta-lactam used as monotherapy.46,47 The use of combination therapy (typically beta-lactam plus aminoglycoside) has shown to be associated with higher toxicity and no superior outcome. Interestingly, however, an Australian survey in 2009 showed that almost half the doctors would choose a combination regimen when presented with a low-risk inpatient with fever and neutropenia,48 suggesting that physicians may be more concerned with the possibility of failure of the antibiotic regimen than with toxicity. Regarding the choice of beta-lactam, no single agent is clearly superior, although piperacillin-tazobactam compares favorably in terms of equivalent efficacy and less toxicity (as long as the frequency of resistant bacteria is low).10,49 Concerns regarding increased overall mortality with cefepime have been largely dismissed by the IDSA13 and ASCO17 after a reanalysis of the cefepime data by the FDA, although the authors of the original meta-analysis remain unconvinced.49
A significant limitation of the evidence is that randomized trials of antibiotics in neutropenic patients have been performed since the 1980s. Some of the data are more than 20 years old and their applicability in the current microbiological milieu has been questioned. The global epidemiology (eg, increasing frequency of gram-positive isolates) has changed, and the local epidemiology at some centers: (eg, high prevalence of multiresistant pathogens) may make the evidence on which the guidelines are based irrelevant. For example, the newest version of the IDSA guidelines have taken ceftazidime out of the list of “preferred” single agents based on current susceptibility patterns.13 Similar concerns are raised by the NCCN.45 As the guideline-endorsed practice of fluoroquinolone prophylaxis becomes more common, it is possible that the relative efficacy of monotherapy versus combination therapy will again be questioned. There have also been changes in the population of neutropenic patients and their infectious diseases risks. Stem cell transplantation, immunomodulators, and biological agents may all increase the immune compromise. It is reasonable to wonder whether the information acquired in the course of leukemia trials in the 1990s is the best source of evidence to decide on the management of the prolonged neutropenia experienced by the recipient of a cord blood transplantation.51
Persistent fever
Definition.
An episode of fever during neutropenia that does not resolve after 5 days of broad-spectrum antibacterial agents.
Background.
IFI was identified as a common cause of this syndrome in the 1970s.52 Two randomized trials established the benefit of adding amphotericin B (one after 7, the other after 4 days of persistent fever) compared with continuing the antibacterial regimen in terms of preventing fungal infection, although they did not have power to demonstrate a survival advantage.53,54 These 2 studies took place before systemic antifungal prophylaxis with fluconazole was used and approximately half the fungal infections documented were caused by Candida. However, the initiation of antifungal therapy after 4-7 days of fever became standard of care, and most RCTs have focused on the choice of drug (liposomal amphotericin B,55 voriconazole,56 caspofungin57,58 ). To reduce the perceived unnecessary use of empirical antifungal therapy, with its attendant toxicity and cost, an alternative approach to empirical antifungal therapy has been proposed and called preemptive antifungal therapy.59 The goal is to use the currently available diagnostic modalities (CT, serum galactomannan, and/or b-D-glucan) to postpone starting antifungal therapy until IFI is more likely. By design, this approach means that patients receiving “preemptive” antifungals are more likely to actually have an IFI than patients receiving “empirical” therapy by the time the antifungal agent is started.60
Recommendations from the guidelines.
All guidelines recommend thinking about fungal infection as a cause of persistent fever and advise some kind of diagnostic workup (including chest ± sinus CT) directed at identifying or ruling out fungal infection in patients who are at high risk for it (notice that the high risk for fungal infection is NOT the same as the “high risk” identified by a MASCC score < 21). The IDSA considers patients with neutropenia expected to last ≥ 7 days “high risk” and advises to “consider empirical antifungal therapy” for them after 4-7 days of fever.13 The IDSA also endorses the use of the “preemptive” antifungal therapy strategy for selected patients.13 The guidelines generally agree that, if it is decided to initiate empirical antifungal therapy for persistent fever, caspofungin or liposomal amphotericin B are the drugs of choice. They differ somewhat in their assessment of the quality of the evidence.
Highlights and limitations of the evidence.
The true frequency of IFI as a cause of persistent fever varies with the clinical setting, but it seems likely that most patients with persistent fever do not have fungal infection. In the 2 original studies (performed before fluconazole prophylaxis was available), the documented fungal infections in the groups randomized to NOT receiving amphotericin were 6 of 16 patients.53 and 6 of 64 patients.54 Subsequent trials have found even lower rates (< 10%), but this may be an underestimation given that all the patients received antifungal therapy after 96 hours.55-58 Therefore, the superiority of any agent is going to be based on relatively few true cases. Because it is unknown how frequent breakthrough fungal infection truly is in neutropenic patients who are receiving systemic antifungal prophylaxis, it is impossible to be certain whether the empirical substitution or addition of another antifungal agent (and which one) is the best possible strategy. Regarding the preemptive approach, the few studies published so far seem promising.60,61
Recurrent or recrudescent fever
Definition.
Recurrent fever refers to a new episode of fever that takes place after the initial episode has resolved with antimicrobial therapy when the patient remains neutropenic (“recrudescent neutropenic fever syndrome”24 ).
Background.
Recurrent or recrudescent fever is a relatively common occurrence in clinical practice that has not been adequately studied, presumably due to the logistical difficulties associated with designing a RCT for a very heterogeneous patient population. The one systematic investigation of this syndrome analyzed data on 836 neutropenic patients who had had a first fever that responded to antimicrobials and then had remained afebrile for 4 days.62 A total of 129 (15%, confidence interval 13%-18%) of the 836 patients developed a second episode of fever or infection. There were 40 bacterial/fungal microbiologically documented infections (15 of them fungal), 11 viral infections, 39 clinically documented infections, and 39 cases of fever of unknown origin. Other small series support the notion that both bacterial and fungal infections are common causes of this syndrome.63 The relative likelihood of one versus the other probably varies with the clinical scenario.
Recommendations from the guidelines.
The various guidelines do not address this clinical circumstance separately from persistent fever. They usually include recommendations for “persistent or recurrent fever” (ie, diagnostic workup for IFI and addition/modification of the antifungal regimen), but these 2 may be clinically and etiologically different situations. Expert advice includes modifying antibacterial and antifungal therapy to cover potential “holes” in the coverage.24
Highlights and limitations of the evidence.
There is no good-quality evidence regarding the management of recrudescent fever.
Engraftment fever (myeloid reconstitution syndrome)
Definition.
Bow defined this syndrome as “new onset or worsening of clinically or radiologically appreciable foci, consistent with an inflammatory and/or infectious process, in temporal relationship to neutrophil recovery after aplasia.”24
Background.
The 3 likely causes of this situation are superinfection, immune reconstitution syndrome, and engraftment syndrome. Apparent worsening of pulmonary aspergillosis has been described at the time of neutrophil recovery,64 so it has been postulated that this situation represents a variation of the “immune reconstitution syndrome” well characterized in AIDS patients after starting effective antiviral therapy. However, fever at this time may also represent superinfection (like the recurrent/recrudescent fever) or, particularly in the setting of stem cell transplantation, a manifestation of engraftment syndrome, a cluster of signs and symptoms including fever, rash, and pulmonary infiltrates originally described after autologous stem cell transplantation but also seen with variable frequency after allogeneic transplantation.65
Recommendations from the guidelines.
This syndrome is not addressed by any of the guidelines.
Limitations and highlights of the evidence.
There is no good evidence regarding the diagnosis or management of engraftment syndrome, which is usually treated with corticosteroids when severe. Diagnostic criteria have been proposed, but not externally validated. A basic point is that engraftment syndrome is a diagnosis of exclusion.
Summary and conclusion
Clinical research on FN over the last 4 decades has generated an impressive body of evidence that allows strong recommendations for the 2 more stereotypical clinical situations: initial fever and persistent fever. However, as the course of the neutropenic episode extends over time and the complexity of the patient increases, there is an increase in uncertainty and obtaining generalizable evidence becomes more difficult. For example, many patients with persistent fever may already be receiving antifungal agents with activity against Aspergillus. What is the best therapeutic strategy in this situation? Similar unanswered questions include the best management of fever in patients who are receiving levofloxacin prophylaxis, the approach to recrudescent (as opposed to persistent) fever, the long-term consequences of the utilization of particular antimicrobial strategies, and the management of patients known to be colonized with resistant pathogens. Finally, the variety of recommendations published by different professional organizations may be confusing. Too many guidelines addressing the issues slightly differently require the reader to become familiar with different grading systems. This issue has been noticed before and the solution is not obvious,66 but the lack of homogeneity in how practice guidelines are formulated has the potential to generate confusion at best and disregard at worst. It seems desirable to develop a common language that will help the individual physician interpret the guidelines appropriately and for this the GRADE system could be a strategy worth considering.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Ceftazidime, meropenem, piperacillin-tazobactam, and voriconazole are not FDA approved for fever and neutropenia. Their use in fever and neutropenia is discussed.
Correspondence
Juan Gea-Banacloche, MD, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Building 10, Hatfield CRC, Room 3-3130, Bethesda, MD 20892-1203; Phone: 301-435-2326; Fax: 301-480-4354; e-mail: banacloj@mail.nih.gov.
