Abstract
Viral infections remain a major cause of morbidity in patients with immunodeficiency, such as recipients of hemopoietic stem cell transplantation. Adoptive transfer of donor-derived virus-specific cytotoxic T lymphocytes is a strategy to restore virus-specific immunity to prevent or treat viral diseases and has been tested in the clinical setting for more than 20 years. Several different groups have used expanded virus-specific T-cell products specific for one or multiple viruses to both reconstitute antiviral immunity after transplantation and to treat active viral infections. Response rates are encouraging, although resistance has been seen when the infused cell population has had restricted specificity or has targeted antigens expressed in donor-infected but not virally infected recipient cells. The goal of current trials is to make this approach more broadly applicable using more rapidly available products from the donor, such as directly selected or briefly expanded cells or closely matched banked cells.
Introduction
Viral infections can be a major cause of morbidity and mortality in patients with immunodeficiency. The role of T cells in controlling viral infections has been clearly demonstrated both in murine and human studies, leading to the exploration of strategies to transfer an antigen-specific immune response to reconstitute immunity and to treat infections.1-3 Most such studies have been undertaken in hemopoietic stem cell transplantation (HSCT) recipients for whom a healthy donor is usually available. In the 2 decades since the first reports that donor-derived CMV-specific cytotoxic T cells (CTLs) reconstituted protective donor immunity to CMV after allogeneic HSCT, several studies including several hundred patients have confirmed activity justifying definitive testing in late phase randomized trials. Recent manufacturing advances have greatly simplified production so that several approaches are now being evaluated in late-phase or licensing studies, raising the prospect that adoptive transfer of virus-specific CTLs may become a standard of care after HSCT. In addition, recent studies showing activity of closely matched third-party cells have opened the possibility of wider application in other patients with immunodeficiency.4,5
CTL generation
Donor requirements
Donors who are seropositive for a particular virus will have virus-specific memory T cells that can be expanded by repeated stimulation with APCs expressing viral antigens. After primary stimulation, which is followed by expansion with cytokines and repeated stimulation with antigen, virus-specific T cells will be expanded and alloreactive T cells should not survive. However, the generation of virus-specific CTLs is a challenge when the donor lacks viral immunity for the infecting virus or with cord blood transplantations in which the immune cells are virus naive.6
Antigens and APCs
To generate virus-specific T cells ex vivo, it is necessary to know the immunogenic antigens for the target virus and have a means of expressing them in an APC with appropriate costimulation to reactivate T cells. The immunodominant antigens have been well defined for the latent herpes viruses such as CMV2 and EBV.7 More recently, several groups have defined appropriate target antigens for other viruses that cause morbidity and mortality after transplantation, such as adenovirus,8 HHV6,9 BK,10 and VZV.11 These immunogenic antigens must be presented on an APCs that expresses MHC antigens along with costimulatory molecules sufficient to support T-cell activation and expansion. In early studies, investigators used virus or virus lysates to expand virus-specific T cells but, more recently, the availability of overlapping peptide pools or pepmixes covering whole viral protein has allowed manufacturing to be simplified and live viruses eliminated.
Ex vivo culture
Initial studies of virus-specific CTLs used prolonged culture for 3-4 weeks and, in some cases, cloning to ensure that alloreactive cells were eliminated. More recently, several investigators have evaluated shorter ex vivo culture periods of between 7 and 14 days, along with the use of newer cytokine combinations to expand virus-specific T cells. For example, Gerdemann et al have used dendritic cells nucleofected with DNA plasmids encoding immunogenic EBV (LMP2, EBNA1, BZLF1), adenovirus (hexon, penton), and CMV (pp65 and IE1) and expanded multivirus CTL lines with the cytokines IL-4 and IL-7.12 Overlapping peptide pools are also used as a source of antigen to further simplify the manufacturing process and to reduce the ex vivo culture time.12-14
Rapid selection strategies
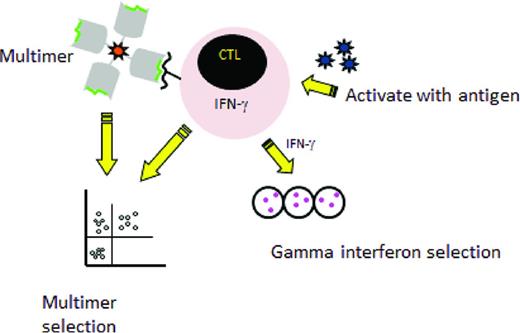
Two major rapid selection strategies are currently being tested in the clinical setting (Figure 1) The first is multimer selection, in which T cells reactive with an immunogenic peptide are selected using magnetically labeled peptide multimers. This strategy is only feasible when the donor has a high frequency of T cells reactive with the viral peptide, so it has largely been confined to the latent herpesviruses CMV and EBV. Results have been encouraging, with massive expansion after transfer of small numbers of T cells15,16 and this approach is now being tested in a phase 3 randomized trial in the United Kingdom to treat CMV reactivation (NCT01077908). An advantage to this method is that the use of multimers avoids the use of an APC, but limitations of multimer selection are the large volume of blood needed, which generally mandates a pheresis; the restriction to HLA alleles for which viral peptides are available and to which the donor has sufficient circulating T cells to allow selection; and the risk of viral evasion when only one peptide is targeted.
Rapid selection procedures. Left: Selection of virus-specific cytotoxic T cells recognizing an HLA-restricted peptide by incubation with an HLA–peptide multimer and then a collection of multimer positive T-cells. Right: Selection by the gamma capture. Donor cells are stimulated with antigen and activated cells that secrete IFN-γ are selected using an immunomagnetic separation device that captures the IFN-γ–secreting T cells specific for the virus.
Rapid selection procedures. Left: Selection of virus-specific cytotoxic T cells recognizing an HLA-restricted peptide by incubation with an HLA–peptide multimer and then a collection of multimer positive T-cells. Right: Selection by the gamma capture. Donor cells are stimulated with antigen and activated cells that secrete IFN-γ are selected using an immunomagnetic separation device that captures the IFN-γ–secreting T cells specific for the virus.
A second selection strategy that has entered clinical testing is IFN-γ capture, in which T cells that respond to stimulation by APCs expressing viral antigens by production of IFN-γ are selected. Trials have been reported using IFN-γ capture to select T cells specific for several viruses, including CMV, EBV, and adenovirus.17-20 Compared with multimer selection, this strategy is not restricted to certain HLA types and the selected population contains both CD4 and CD8 T cells recognizing multiple epitopes, thus lowering the likelihood of virus evasion. However, the manufacturing process still requires large volumes of donor blood.
Clinical results
Extended culture protocols
CMV.
CMV was the first virus targeted by adoptive transfer strategies by investigators in Seattle, who used donor fibroblasts as APCs and the AD169 strain of CMV as a source of antigen to generate CMV-specific CTL lines that were then cloned. Donor-derived CMV-specific CD8+ clones were then infused in a dose-escalation study to recipients of matched sibling donor grafts. The investigators showed that this strategy did not induce GVHD and resulted in reconstitution of CMV-specific CD8+ T-cell responses.21,22 The development of a CD4+ CMV response was required for long-term persistence of the adoptively transferred CD8+ T-cell clones. That study was an important proof of principle demonstrating the potential of adoptive immunotherapy to reconstitute antiviral immunity, but the manufacturing process was complex and prolonged and used live virus.
Several other groups developed different manufacturing strategies to generate CMV-specific CTLs. Einsele et al pulsed donor-derived PBMCs with CMV lysate to expand responding T cells and infused the CTL line to 8 patients who had persistent CMV disease despite receiving pharmacotherapy. Six of 7 evaluable patients cleared viremia long term after 1 or 2 doses of T cells, coincident with an increase in both CD4+ and CD8+ CMV-specific T cells.23 Although the manufacturing process was less cumbersome, the use of CMV lysate confers a risk of infection. Peggs et al used dendritic cells pulsed with CMV antigen derived from a CMV-infected human lung fibroblast cell line, which produced the expansion of a polyclonal CTL product with broad specificity.24 In that study, CMV CTLs were infused to 16 patients at the time of first CMV PCR positivity and none developed CMV disease, although 2 patients had a subsequent CMV reactivation treated with ganciclovir.24 Perruccio et al also used CMV antigen to stimulate CTLs, but then cloned the lines and administered CD4+ CMV-specific T-cell clones to 25 recipients of haploidentical donor grafts.25 The response rate in that study was less impressive, with 7 patients having a CMV reactivation and 5 patients developing CMV disease, which was fatal in 2. This experience argues for the use of a broad polyclonal product containing both CD4+ and CD8+ T cells.
EBV.
Reports of successful treatment of EBV lymphomas arising after HSCT in ∼ 70% of patients who receive donor lymphocyte infusion4 provided a clear rationale for the evaluation of donor EBV CTLs to reduce the risk of alloreactivity. Because 95% of the population is EBV seropositive and the frequency of EBV-specific T cells can be up to 1%, this was an ideal scenario to evaluate the activity of donor-derived CTLs. Unlike other viral infections, EBV lymphomas more commonly arise in donor rather than recipient cells. EBV-specific T cells were generated using repeated stimulations with irradiated lymphoblastoid lines (LCLs) generated by infecting donor mononuclear cells with the B-95 laboratory strain of EBV.26 In a review of 114 patients who received EBV-specific CTLs as prophylaxis or treatment for EBV lymphoma after transplantation in our center, the infused donor cells did not induce de novo GVHD and the main toxicity was inflammatory reactions at disease sites in 4 patients who were treated with bulky disease.27 None of the 101 patients who received the CTL as prophylaxis developed EBV lymphoma, whereas 11.5% of controls developed this complication. Of 13 patients who received EBV-specific CTL when they had established EBV lymphoma, 11 achieved sustained complete remissions. One of the nonresponders revealed an important virus evasion strategy because the donor was HLA A11, which is a dominant restricting allele in the immune response to EBV, so that the infused line predominantly recognized 2 epitopes in EBNA 3B. The patient's tumor had a deletion that removed these 2 immunodominant epitopes, rendering it resistant to the infused CTLs.28
Doubrovina et al at Memorial Sloan-Kettering Cancer Center obtained similar response rates when they infused donor-derived EBV-specific T cells to treat 14 patients with EBV lymphoma, obtaining complete responses in 10.4 They also identified tumor evasion mechanisms when they investigated the reasons for failure. In 3 cases, the infused CTLs recognized the LCLs used to generate the CTL line, but not the patient's tumor cells or spontaneous LCL lines established from the patients' blood. Along with the case above, this experience shows that antigenic differences between EBV strains in patients and the laboratory B95-8 strain used to transform LCLs for use as APCs can be significant. In the other patient, the donor was A1101 and the CTL line was skewed in its response to EBV peptides presented by this antigen, which in this case was not present in this recipient.4 These experiences illustrate the importance of administering a broad immune response to ensure that the CTL line contains specificity against peptides expressed by the tumor's HLA antigens.
Multivirus.
To cover a wider range of viruses, our group developed a strategy to generate trivirus-specific donor-derived T-cell lines recognizing CMV, EBV, and adenovirus.29 In CMV-seropositive donors, we used an adenovirus vector expressing the CMVpp65 protein (Ad5f35-pp65) to modify monocytes and EBV-LCLs to generate a single culture of CTLs specific for EBV, CMV, and adenovirus; if the donor was CMV seronegative, we used an empty adenoviral vector that produced CTLs specific for EBV and adenovirus. We infused the trivirus-specific CTL products to 26 patients and observed immune reconstitution to CMV and EBV, but could only detect immune responses to adenovirus in patients with a reactivation or infection. Ten of the 11 patients with CMV reactivation cleared CMV viremia in association with an increase in CMV-specific CTL in peripheral blood, as did 6 of 6 patients with high EBV loads and 5 of 6 patients with adenovirus infections.29 Of the 14 patients who received bivirus-specific CTLs after HSCT, 3 of 3 patients with high EBV loads cleared their virus, as did 2 of 3 patients with adenoviral infections/disease.30 There was no infusion-related toxicity and no patient developed > grade II GVHD in either study.
Blyth et al have recently reported a phase 2 study of 50 allogeneic HSCT recipients in which 40 patients received donor-derived CMV and adenovirus generated by stimulation with the same chimeric adenovirus-CMVpp65 vector, but in this case expressed in dendritic cells for all stimulations.31 When the investigators compared outcomes against concomitant controls who did not receive CTLs, they observed no significant difference in the incidence of acute or chronic GVHD or in the rate of CMV reactivation, but there was a significant reduction in the number requiring pharmacotherapy and in the duration of such therapy in the CTL cohort.31
Clinical results with rapid CTLs
The results of the trials described above and others showed that donor-derived virus-specific CTL therapy is safe and effective when used either as prophylaxis or as treatment for infections with several viruses after HSCT. However, although the overall response rates were encouraging, the manufacturing methodology was complex, and broader use was limited by the 2- to 3-month time frame time required for the generation of virus-specific CTLs. In addition, more recent strategies have attempted to move away from the use of live virus or whole viral antigens.
Multimers.
In the first trial to use the multimer approach, 9 patients received products selected with CMV tetramers containing a median dose of 8.6 × 103 CMV tetramer-staining CTLs/kg. These small numbers expanded by several logs in vivo and 8 of 9 patients cleared the viremia, including one patient with drug-resistant disease.15 Schmitt et al have also used the streptamer technology to select pp65-specific T cells and successfully cleared CMV reactivation in 2 patients.16 Multimer selection from a haploidentical donor has also been used to treat EBV lymphoma developing in a recipient of a cord blood transplantation. HLA A2-restricted T cells specific for epitopes in 2 EBV antigens were selected and infused, resulting in a clinical response.32 The EBV lymphoma recurred several months later, but a second infusion also produced a response.32 Therefore, multimer-selected cells have activity, but the strategy is limited to a small numbers of donors who have sufficient circulating T cells reactive with a known HLA-restricted viral peptide epitope.
IFN-γ capture.
CMV has been the major target of approaches using IFN-γ capture. Feuchtinger et al used pp65 protein as a source of antigen, infusing CMV-specific T cells isolated by IFN-γ capture to 18 patients with CMV disease or viremia, and reported responses in 83%.18 Peggs et al used recombinant CMV pp65 protein or overlapping peptides derived from the CMV pp65 to stimulate donor pheresis products and infused isolated CMV-specific T cells to 25 HSCT recipients, resulting in reconstitution of CMV immunity.33 Meij et al used 2 immunodominant CMVpp65 peptides restricted by HLA-A*0201 or HLA-B*0702 to induce CMV CTLs and achieved complete responses after infusion in 6 patients with CMV reactivation.34
The IFN-γ capture technology has been used to select donor-derived EBV-specific T cells to treat patients with EBV lymphoma. Moosmann et al used 23 class I and II peptides derived from 11 EBV antigens as a source of antigen and peripheral blood cells as the APC, and then selected T cells secreting IFN-γ.17 Three of 6 patients infused responded, whereas the other 3 patients who had more advanced disease failed to respond.17 Icheva et al used whole EBNA-1 protein or EBNA-1 overlapping peptide pools as a source of antigen to stimulate EBNA-1–specific T cells for the selection by IFN-γ capture. These T cells targeting just one EBV antigen, EBNA-1, induced remission in 7 of 10 patients with EBV lymphoma.35 IFN-γ capture has also been used to selected adenovirus-reactive T cells by simulation of donor PBMCs with adenoviral antigen.19 The lower frequency of adenovirus-specific T cells was a limitation, but 4 of 9 pediatric patients with systemic adenoviral infection after HSCT cleared the virus.19
The results of these studies show that cells selected by IFN-γ capture have clinical activity, although it is still unclear whether there is increased incidence of failure due to immune escape when only targeting a limited number of antigens and/or epitopes. Further, the number of donor virus-specific cells may be limiting for viruses other than CMV or EBV without longer ex vivo culture.
Short ex vivo culture.
An alternative to immediate selection is to use short ex vivo culture. Rapid multivirus CTLs generated using dendritic cells nucleofected with DNA plasmids have been administered to 10 allogeneic HSCT recipients, with a response rate of 80%, including one patient with a biopsy-proven EBV lymphoma and 3 patients with double viral reactivations.36 Several studies using overlapping peptides as a source of antigen with optimized cytokines in a short ex vivo culture are under way.
Third-party cells
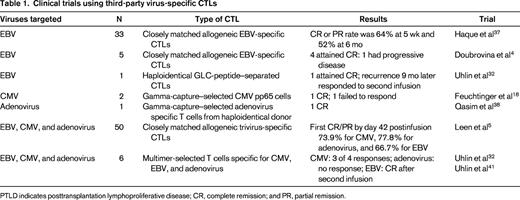
With current manufacturing methodologies, it is difficult to manufacture CTLs from virus-naive donors, although preclinical studies are evaluating the use of different cytokine conditions.6 One option that could provide an “off-the-shelf” product to all recipients is to use banked virus-specific T-cell lines generated from virus-immune subjects with common HLA polymorphisms; studies that have tested this approach are summarized in Table 1. The first study to evaluate this approach was reported by Haque et al, who used banked polyclonal EBV CTL lines to treat EBV lymphomas that had failed to respond to standard treatments after HSCT or solid organ transplantation, reporting an overall response rate of 52% at 6 months.37 In a report from Memorial Sloan-Kettering, complete responses were obtained in 4 of 5 patients with EBV lymphoma who received third-party EBV CTL lines.4 More recently, a multicenter study evaluated the use of third-party multivirus-specific CTLs to treat patients with refractory viral infections and reported an overall cumulative incidence of first CR/PR by day 42 postinfusion of 73.9% for CMV, 77.8% for adenovirus, and 66.7% for EBV.5 Although these response rates are slightly less than those seen with donor-derived CTLs, there is significant activity. Apart from one report of bystander-induced liver GVHD after third-party adenovirus CTLs,38 there does not seem to be an increased risk of alloreactivity. It is not yet clear what the optimal criteria are for selecting a matched line and what factors are associated with failure to respond. Possible reasons for failure include the presence of HLA antibodies reactive with the infused line or insufficient activity against the infecting virus through shared alleles.
Use of viral CTLs after solid organ transplantation
Adoptive immunotherapy with virus-specific CTLs has also been evaluated to treat viral infections after solid organ transplantation. Several studies have infused autologous EBV-specific CTLs generated from peripheral blood of the recipient, with responses reported in some patients.39 However, long-term EBV-specific immunity has not been obtained, likely because solid organ transplantation recipients continue to receive immunosuppression. Infusion of third-party CTLs derived from a bank of EBV-seropositive donors resulted in a 52% response rate at 6 months in patients who had received solid organ transplantation or HSCT.37 An update 4 to 9 years after CTL infusion showed an increased survival among recipients who attained a partial or complete response. Of the 14 patients who achieved an initial complete response, only 1 subsequently died of relapse.40 A concern with infusion of virus-specific CTLs after solid organ transplantation is that infused CTLs may also react against the transplanted organ, inducing rejection, but this has not been observed.
Conclusions
In summary, these clinical studies show that both donor-derived and third-party virus-specific T cells targeting 1 to 3 viruses have efficacy in the post-HSCT setting and can reconstitute immunity and clear viral reactivations and disease. The incidence of GVHD did not appear to be increased over that which would be expected in the patient population. Overall, the response rates in studies of donor-derived virus-specific CTLs to treat viral reactivation or disease have ranged from 70% to 100%, whereas response rates with third-party cells have ranged from 60% to 80%. Several causes of viral evasion have been shown, and it appears important to infuse a product with broad reactivity and to ensure that the infused line contains antiviral activity restricted by HLA antigens expressed on the virally infected cell. The more widespread testing of this strategy has been limited by the complexity of some of the CTL-manufacturing methodologies and the lengthy production time. Over the past few years, several groups have developed more rapid CTL generation protocols that appear to have equivalent activity, allowing definitive testing in late-phase trials. It will be important to design such studies with appropriate end points, standard criteria for instituting and stopping antiviral drugs, and including comparative effectiveness analyses.
Disclosures
Conflict-of-interest disclosures: H.E.H. holds patents with or receives royalties from Cell Medica and Celgene. A.M.L. has received research funding from Celgene. Off-label drug use: IND cell therapy products.
Correspondence
Dr Helen Heslop, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1640, Houston, TX 77030; Phone: 832-824-4662; Fax: 832-825-4668; e-mail: hheslop@bcm.edu.