Abstract
Patients with acute myeloid leukemia who harbor an FMS-like tyrosine kinase 3 (FLT3) mutation present several dilemmas for the clinician. The results of an FLT3 mutation test, which can be influenced by several variables, need to be interpreted according to the clinical setting and there is a need for internationally standardized FLT3 mutation assays. Because of the lack of prospective studies, the role of allogeneic transplantation as consolidation therapy is still somewhat controversial, but the preponderance of evidence suggests that transplantation in first remission, if possible, is probably the best option. Clinically useful FLT3 inhibitors are hopefully on the near horizon and are being studied in the context of current treatment paradigms.
Introduction
More than for almost any other malignancy, therapy for patients with acute myeloid leukemia (AML) is guided as much by the molecular and cytogenetic profile as it is by the patient profile.1 Mutations in the FMS-like tyrosine kinase 3 (FLT3) gene represent one of the most frequently encountered, and clinically challenging, class of AML mutations.2 Although approximately 30% of AML patients harbor some form of FLT3 mutation, the clinical significance one of these genetic lesions in any given patient varies according to the nature of the mutation and the context in which it occurs. In general, FLT3 mutations can be divided into 2 categories: (1) internal tandem duplications (FLT3/ITD mutations) in or near the juxtamembrane domain of the receptor and (2) point mutations resulting in single amino acid substitutions occurring within the activation loop of the tyrosine kinase domain (FLT3/TKD mutations). The incidence of FLT3/ITD mutations (Table 1) varies according to age and clinical risk group, being less common in pediatric AML and in AML arising from an antecedent myelodysplastic syndrome.3-17 There is less of a clear pattern with FLT3/TKD mutations, which are reported to occur in approximately 7% of patients, although they seem to be more common in cytogenetically favorable risk AML.7,18-21
Incidence and prognostic impact of FLT3/ITD mutations in AML subgroups
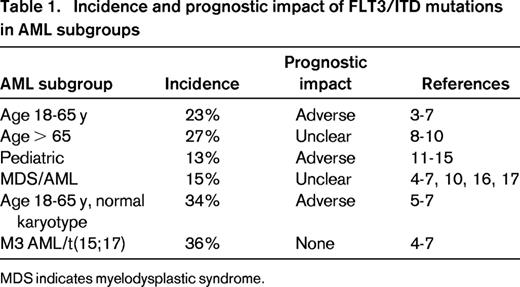
MDS indicates myelodysplastic syndrome.
FLT3/ITD mutations (which occur in approximately a quarter of newly diagnosed adult cases) invariably present the greatest clinical challenge. Typically, patients with FLT3/ITD AML, particularly those with normal or intermediate risk karyotype, present with a very large disease burden manifesting as leukocytosis and a packed BM. In adults under age 60 to 65 years with non-M3 FLT3/ITD AML, remission can be achieved with conventional induction therapy with a frequency similar to other AML patients, but remission durations are shorter and relapse rates are higher. Once relapsed, the disease is rapidly fatal. FLT3/TKD mutations are less common (∼ 7%) and do not have a characteristic clinical signature.18,19 Although their prognostic impact is much less evident than the FLT3/ITD mutations, they may ultimately prove to be very significant clinically because they represent an important mechanism of resistance to FLT3 tyrosine kinase inhibitors (TKIs).22 Both classes of FLT3 mutations constitutively activate the receptor, but the mechanism of activation is different for each, which probably accounts for the biological differences observed between the 2 classes. FLT3/ITD mutations are in-frame duplications that invariably involve the juxtamembrane domain, a domain that exerts a negative regulatory function over the kinase activity.23 The autoregulatory function of the juxtamembrane region is a common structural motif of receptor tyrosine kinases and, whereas the kinase domain itself remains intact, signaling is constitutive and aberrant, possibly because of the addition of duplicated tyrosine residues.24 The duplicated portion in most cases encompasses residue arginine 595, but can extend well into the kinase domain.25 In the examples of these longer insertions, the duplicated sequence actually starts within the kinase domain and may well result in an even worse prognosis than shorter insertions.26 In contrast, FLT3/TKD mutations occur at or near the active site, most commonly at aspartate 835 (structurally analogous to the commonly mutated aspartate 816 residue in c-KIT) but also at aspartate 842 or isoleucine 836.18 Several different amino acid substitutions have been identified and there are structural data from related receptors to suggest that these mutations have a very different mechanism of receptor activation compared with the FLT3/ITD mutations, perhaps accounting for the differences in biologic and clinical effect.27
Recently emerging data from whole-genome sequencing studies of patient specimens indicates that, at diagnosis, AML is polyclonal, but a dominant clone tends to emerge at relapse.28 An FLT3/ITD-containing subclone, therefore, potentially represents only one clone amongst several. In most cases, the clone or clones that emerge at relapse continue to harbor the FLT3/ITD mutation and, moreover, the leukemia cells appear to be more “FLT3 addicted” with regard to in vitro response to FLT3 inhibition.29,30 Occasionally, an FLT3/ITD mutation detected at diagnosis is undetectable at the time of relapse, indicating that the clone was either eliminated by chemotherapy or simply failed to re-expand from the pool of persisting leukemia stem cells.
Clinical dilemmas in the management of FLT3-mutated AML
Currently, the clinical dilemmas that face a practitioner treating an AML patient with an FLT3 mutation center around the interpretation of FLT3 mutation test results and around the decisions on the best induction and consolidation therapy. In addition, FLT3 TKIs are under active clinical investigation and whereas there are some data supporting off-label use of TKIs for FLT3/ITD AML, the decision to refer a patient for a trial of such agents, or to use these drugs off-label, represents yet another dilemma.
FLT3 mutation testing
PCR assays for FLT3 mutations
Clinically validated FLT3 mutation testing is performed with a PCR-based assay of genomic DNA isolated from a sample of the patient's leukemia cells. The most commonly used assay, which is available in commercial laboratories and in the certified laboratories of several tertiary care centers, uses genomic DNA prepared from whole blood or BM. The number of blasts within the sample tested is crucial because samples diluted with nonmalignant hematopoietic elements will lower the sensitivity of the assay. The most common assay method used is that reported by Murphy et al, which is actually a duplex assay testing for both types of FLT3 mutations in the same tube: PCR primers (labeled with a fluorochrome) flanking the juxtamembrane coding sequence amplify FLT3/ITD mutations, whereas a second set of primers amplify the kinase domain sequence in the same reaction tube.31 The amplified fragments are separated using capillary gel electrophoresis and the fluorochrome signals are analyzed using a software program such as GeneScan. An EcoRV digestion of the PCR products will cleave the kinase domain fragment unless a TKD mutation at D835 (or I836) is present. It is important to note that neither PCR product is sequenced; the assay simply determines whether an FLT3/TKD mutation is present or, in the case of FLT3/ITD mutations, the length of the insertion. Because the prognostic effect of FLT3/TKD mutations remains unresolved, some laboratories only assay for FLT3/ITD mutations.
Limitations of the FLT3/ITD mutation assay
A major limitation of most PCR assays for FLT3/ITD mutations is their relative lack of sensitivity, at least compared with PCR assays for other AML-associated lesions such as AML-ETO. Gene fusions such as AML-ETO or BCR-ABL generate a unique sequence highly amenable to amplification over background. The sensitivity of these assays is simply a function of the amount of sample DNA and the number of PCR cycles. In the FLT3/ITD assay, however, increasing the number of cycles will not increase the sensitivity because the PCR primers used to amplify the mutant allele also amplify the wild-type allele. This would not be a problem if both mutant and wild-type alleles were amplified equally, but in this assay, the shorter wild-type allele has a competitive advantage. In a reaction using a typical patient specimen that is heterozygous for an FLT3/ITD mutation, the fragments generated in the PCR reaction consist of the 330-bp wild-type fragment and the longer mutant fragment, the length of which is increased over the wild-type length due to the inserted sequence. The increased length of the mutant sequence translates into a longer time required to complete a PCR cycle, which leads to the wild-type allele being amplified at a more rapid rate than the mutant allele. The result of this wild-type “PCR bias” is that even in a pure 1:1 mixture of mutant and wild-type templates, the mutant-to-wild-type allelic ratio will be less than 1 (ie, the wild-type allele is overrepresented). The longer the insertion is, the greater the PCR bias. For example, an insertion of 120 bp will artificially lower the ratio to a greater degree than a 30-bp insertion. This bias can be minimized using fewer cycles of PCR (eg, 25-27), but this could affect sensitivity when there is a low burden of leukemia cells in the sample.7
FLT3/ITD mutation length
A few studies have examined the impact of the length of mutation on clinical outcome. The median length of a mutation in these studies ranged from 39 to 54 bp, and longer mutations are usually (but not always) found to be associated with reduced remission rate and/or worse overall survival.26,32 The structural consequence of a long duplication of a sequence beginning at or around the codon for arginine 595 is that the actual duplicated sequence starts within the kinase domain rather than within the juxtamembrane domain.26
Allelic ratio
For FLT3/ITD mutations, the allelic ratio refers to the number of ITD-mutated alleles compared with the number of wild-type allele. This ratio is not only influenced by the amount of malignant versus nonmalignant cells in the sample tested, but also by the percentages of cells with 0, 1, or 2 mutated alleles. AML is a polyclonal disease at presentation28 and, whereas in most cases, the dominant cell in a diagnostic sample is heterozygous for the mutation, subpopulations within the sample can lack the mutation altogether or be biallelic (uniparental disomy).33 Still other cells can be hemizygous for the mutant allele34 either through outright loss of the chromosome 13 containing the wild-type allele (which is identifiable using conventional cytogenetic analysis) or through a smaller deletion of the wild-type (detectable using a single-nucleotide polymorphism array).
As FLT3/AML evolves from diagnosis to relapse, the mutant to wild-type allelic burden also typically evolves.29,35 Occasionally, the mutation is completely absent (or at least undetectable) at relapse. This typically is seen in cases in which the mutant allelic burden is low (eg, 5%-15%) at diagnosis. This phenomenon, coupled with the overall lack of sensitivity of the assay, is why FLT3/ITD mutations have been regarded as unsuitable for use as a marker of minimal residual disease. In most cases, however, the mutation originally detected at diagnosis is also present at relapse, and often at an even higher allelic ratio than at diagnosis. Several studies in which FLT3/ITD mutation testing was performed on banked specimens from clinical trials have concluded that higher mutant to wild-type allelic ratio is predictive of worse outcomes, with the worst scenario of all being loss of the wild-type allele altogether.7,34 The negative prognostic impact of a high mutant allelic burden hold true even in studies that have modified the assay to account for the PCR bias from mutation length.7,36 In the context of the polyclonal nature of AML at diagnosis, the allelic ratio is to some degree a reflection of the clonal burden of the FLT3/ITD-mutated cells within the leukemia cell population. As AML evolves from diagnosis to relapse and a dominant clone emerges, the allelic ratio tends to rise.
Interaction with other genetic lesions
The prognostic impact of an FLT3 mutation must be interpreted according to its genetic context. FLT3/TKD mutations, for example, seem to occur more frequently in cases with INV,16 although the prognostic impact is uncertain.20,21 Although FLT3/TKD mutations seem to have little impact in any context, the impact of FLT3/ITD mutations can vary considerably in different settings. FLT3/ITD mutations are common in acute promyelocytic leukemia (APL) and although they are associated with leukocytosis (and possibly increased early mortality), their overall impact is minimal in this subtype.37 They occur at a relative low frequency with almost any other cytogenetic abnormality, except t(6;9),7 but their prognostic impact in most situations is negative. FLT3/ITD mutations are most common in cytogenetically normal AML and typically co-occur with either an NPM1 mutation or a DNMT3a mutation.38,39 Retrospective studies of banked clinical samples suggest that the presence of an NPM1 mutation may mitigate the negative prognostic effect of an FLT3/ITD mutation, but possibly only if the FLT3/ITD allelic ratio is low.36,38
Conclusions about FLT3 mutation testing
To summarize, FLT3/ITD mutation testing should be performed in all AML patients at diagnosis for prognostic purposes and for guiding therapeutic decisions, but currently has little utility for minimal residual disease monitoring. It is relatively insensitive compared with other PCR assays and the results are dependent upon the blast percentage assayed and (depending on the number of PCR cycles used) on the length of the insertion. FLT3/ITD mutations are common in AML and, apart from APL, they usually have a pronounced negative impact on clinical outcomes at all stages of the disease. The clinician may be tempted to declare a patient intermediate risk when the mutant-to-wild-type allelic ratio is low (and when there is a coexisting NPM1 mutation), but such a classification should take PCR methodology into account. A safer default at this time, therefore, is still probably to classify all non-APL FLT3/ITD cases as generally poor risk and tailor therapy accordingly. The variations in results from different FLT3/ITD mutation testing methods argue for the development of an international standard to avoid some of the problems encountered with other oncogene testing (such as for BCR-ABL). This will be particularly important as targeted therapies against FLT3 are investigated.
FLT3/ITD AML: how to treat?
A relapse waiting to happen
Newly diagnosed FLT3/ITD AML patients in the age range of 18 to 65 years receiving intensive cytarabine-based induction therapy generally achieve remission at or near the same rate as other AML patients. The exception to this rule comes from those patients presenting with a high allelic burden or who are hemizygous for the mutation: these patients are often refractory to induction.34 Regardless, a propensity to relapse—and relapse quickly—is the salient feature of FLT3/ITD AML patients in first remission. Their median time to relapse is 6 to 7 months compared with 9 to 11 months for patients with other AML subtypes. FLT3/ITD AML at relapse is an extremely dire situation. Although survival for AML patients in first relapse is generally poor, those with an FLT3/ITD mutation can be distinguished as having the shortest survival of any of them.40,41 This is presumably a reflection of the fact that the salvage rate for FLT3/ITD AML in first relapse is lower than in nonmutated cases (22% vs 64% in one study41 ), and is probably also due to the generally aggressive, proliferative nature of the disease. In one trial of FLT3-mutated patients in first relapse treated with conventional salvage chemotherapy (high-dose cytarabine or mitoxantrone/etoposide/cytarabine), only 11% of patients with a first remission duration of 6 months or less achieved a second remission.42
Role of allogeneic transplantation
These dire statistics starkly frame the dilemma facing a clinician deciding on a consolidation therapy for an FLT3/ITD AML patient in first remission: to transplant or not to transplant? For FLT3/ITD AML, the role of allogeneic transplantation as consolidation therapy has been a controversial issue.43 In most studies of AML patients, allogeneic transplantation confers a lower relapse risk compared with conventional chemotherapy. Given that relapse risk is a central feature of FLT3/ITD AML, allogeneic transplantation would seem the logical consolidation choice if not for the fact that transplantation is typically associated with at least a 20% treatment-related mortality risk. Although there have as yet been no prospective studies to specifically address the role of allogeneic transplantation in FLT3/ITD AML, several retrospective analyses have been performed and a coherent picture is emerging.
Gale et al first reported the results of an analysis of 2 clinical trial populations in the United Kingdom in which patients were segregated into donor and no-donor groups and retrospective testing for FLT3/ITD mutation was performed.44 They were unable to establish evidence of benefit for allogeneic transplantation for these patients, but were careful to note that this did not constitute evidence of a lack of benefit. The limitations of the study were that only 35 of 68 FLT3/ITD AML patients with a donor actually underwent transplantation in first remission, and the treatment-related mortality for these patients was 40%. In a study of AML patients with normal karyotype from 4 clinical trial populations in the German-Austrian Acute Myeloid Leukemia Study Group, the investigators concluded that the only patients who did not benefit from allogeneic transplantation as consolidation were those with NPM1 mutations or CEBPA mutations who lacked an FLT3/ITD mutation.45 The conclusion by inference, therefore, was that patients with FLT3/ITD AML should receive transplantation. Several other smaller studies since published have also concluded that allogeneic transplantation conferred a benefit over chemotherapy for FLT3/ITD AML patients (Table 2).43,46-48 Finally, a recent study of cases registered in the European Group for Blood and Marrow Transplantation (EBMT) compared outcomes for 120 FLT3/ITD AML patients undergoing a myeloablative allogeneic transplantation in first remission versus 86 cases lacking the mutation.49 The conclusion of this study is probably consistent with what can be drawn from the other studies: patients with an FLT3/ITD mutation who undergo transplantation in first remission probably have a better outcome than FLT3/ITD AML patients treated with conventional consolidation chemotherapy, but still have a higher relapse risk than transplanted patients lacking the mutation.
Published studies examining the role of allogeneic transplantation in first remission for patients with FLT3/ITD AML

All of these studies are retrospective analyses.
*Number of patients in the study with a FLT3/ITD mutation.
†Whether the authors of the indicated study concluded that transplantation in first remission was beneficial specifically for patients with a FLT3/ITD mutation.
Despite the lack of prospective studies, the clinician must still decide on a course of action. The available data currently support the conclusion that an FLT3/ITD AML patient in first remission should be offered an allogeneic transplantation as consolidation, assuming that a donor can be found and that the patient is an acceptable candidate for the procedure (in terms of age and comorbidities). Given the rapidity with which these patients relapse, it seems prudent to initiate HLA typing and a donor search immediately upon identifying an AML patient as having an FLT3/ITD mutation.50,51 At this time, there are no reliable data available regarding the relative benefits of myeloablative versus reduced-intensity conditioning for these patients.
Elderly patients
Although the maximum age for allogeneic transplantation continues to rise with the use of reduced-intensity conditioning, AML remains a disease most often encountered in the elderly. Therefore, there remains a significant fraction of FLT3/ITD AML patients ineligible for transplantation because of their age. Such patients are often unsuitable for intensive induction therapy and referral to a clinical trial, if possible, is recommended. FLT3 inhibitors have been under active investigation for several years now, either administered as single-agent therapy or in combination with other agents.52 The hypomethylating agents (azacitidine and deoxy-5-azacitidine) have shown activity in newly diagnosed elderly AML patients.53,54 A recent trial in which azacitidine was combined with the tyrosine kinase inhibitor sorafenib (which has activity as a single agent in FLT3/ITD AML) showed promising results,55 and other trials of hypomethylating agents combined with FLT3 inhibitors are under way.
FLT3 inhibitors
Small-molecule FLT3 TKIs have been studied for the past decade, but as yet no agent has been specifically approved for this use. Multitargeted TKIs or TKIs developed for use against other kinase targets have been tested in trials for FLT3/ITD AML, but the consistent theme emerging from these studies has been one of incomplete in vivo FLT3 inhibition.56 Profound, sustained inhibition of FLT3 activity is necessary to induce a cytoxic effect,57 and most of the agents studied have many off-target effects at the doses necessary for complete FLT3 inhibition. Because of the relative lack of in vivo potency of these older agents, clearance of BM blasts is rarely achieved with them as single agents, so they have been and continue to be studied in combination with conventional chemotherapy.42,58,59 Two phase 3 trials of such agents given in combination with induction chemotherapy (lestaurtinib and midostaurin) in newly diagnosed FLT3/ITD AML patients have recently completed accrual, although final results are not yet available.
An important newcomer to the otherwise crowded field of FLT3 inhibitors is quizartinib, which is considerably more potent than any other agent and has the requisite selectivity to be tolerable at doses that completely inhibit FLT3 in vivo.60,61 In a phase 2 trial including a total of 191 relapsed/refractory FLT3/ITD AML patients, 2 cohorts were treated with quizartinib as single-agent therapy.62,63 The first cohort consisted of 92 mostly older patients (median age 69 years) who were relapsed or refractory to a single line of therapy. The second cohort consisted of 99 somewhat younger patients (median age 55 years) who had relapsed or were refractory after 2 lines of therapy. Responses to quizartinib consisted of clearance of peripheral blood blasts and reduction of BM blasts, often to < 5%. Because most patients remained platelet and red cell transfusion dependent, the responses were not considered classic working group remissions but, rather, complete remission with incomplete count recovery (although a few bona fide complete remissions were achieved). The composite remission rate was 51% (98/191). Correlative data from this trial indicated that responses were associated with rapid apoptosis of circulating blasts coupled with the induction of terminal differentiation of BM blasts over the course of a few weeks.64 The dramatic reduction in BM blast count induced by this drug without systemic toxicity allowed 47/136 (35%) cohort 2 patients to undergo allogeneic transplantation, resulting in a significant number of long-term survivors from this very poor-risk group.
Another significant finding that emerged from this trial was that patients achieving a response to quizartinib often developed resistance-conferring point mutations, most commonly at D835 and less frequently at a “gatekeeper” residue, phenylalanine 691 (F691).22 Although this result provided further biologic evidence that FLT3/ITD mutations were true drivers of this disease, it obviously was very problematic clinically and has spurred development of new TKIs with activity against these new mutants.
If an FLT3 inhibitor were ever to be approved, how should it be used? Trials of these agents have enrolled primarily relapsed or refractory patients only. However, FLT3/ITD AML cells from newly diagnosed patients do not seem to be “addicted” to FLT3 signaling, at least not in vitro.30 This may relate to the polyclonal nature of the disease when it first presents.28,33 Therefore, a selective FLT3 inhibitor would be predicted to affect only a subset of the leukemia at diagnosis and therefore would have little use as a single agent in this setting. However, it could be used to suppress the relapse of the FLT3/ITD clone once remission is achieved or to help a high-allelic-ratio patient achieve remission when they would otherwise not. A paradigm for the use of such an agent in FLT3/ITD AML might be derived from the approach used with BCR-ABL inhibitors in the treatment of Philadelphia chromosome–positive acute lymphoblastic leukemia. The TKI could be added to the induction chemotherapy to maximize the remission rate and to maintain the remission long enough to allow the patient to be taken to allogeneic transplantation. After the transplantation, the TKI could be used as maintenance therapy.
Off-label use of FLT3 inhibitors
Three multitargeted TKIs currently approved for other malignancies have in vitro activity against FLT3: sunitinib, sorafenib, and ponatinib.65-67 Sorafenib has been reported to have clinical activity in relapsed FLT3/ITD AML, particularly when relapse occurs after allogeneic transplantation.68,69 Although these reports do not constitute clinical trial evidence of efficacy, they reflect a growing consensus in the community that such agents offer meaningful benefit to a patient population that lacks any effective approved therapies. Nonetheless, it is strongly recommended that, whenever possible, patients with relapsed/refractory FLT3/ITD AML be referred for enrollment one of the numerous ongoing clinical trials of FLT3 inhibitors (Table 3). Regulatory approval of one or more of these agents will require the completion of large, well-designed pivotal studies.
Current FLT3 inhibitor trials
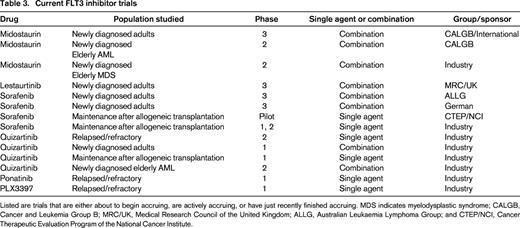
Listed are trials that are either about to begin accruing, are actively accruing, or have just recently finished accruing. MDS indicates myelodysplastic syndrome; CALGB, Cancer and Leukemia Group B; MRC/UK, Medical Research Council of the United Kingdom; ALLG, Australian Leukaemia Lymphoma Group; and CTEP/NCI, Cancer Therapeutic Evaluation Program of the National Cancer Institute.
Conclusions
Optimal management of an AML patient with an FLT3 mutation requires an understanding of the nature of the FLT3 mutation test and of the prognostic impact of the mutations in the context of other genetic lesions and a strong argument could be made that the assay for both types of mutations should probably be standardized internationally. FLT3/ITD AML is a distinct and important subgroup of AML with fairly predictable clinical features. At the present time, allogeneic transplantation seems to be the best option for consolidating younger patients, whereas elderly patients unfit for intensive therapy should be referred for clinical trials whenever possible. The hope is that the incorporation of FLT3 TKIs into this treatment paradigm will lead to a significant improvement in the prognosis for all of these patients.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Ambit Biosciences. Off-label drug use: Off-label use of sorafenib as an FLT3 inhibitor.
Correspondence
Mark Levis, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, 1650 Orleans St, Rm 2M44, Baltimore, MD 21287; Phone: 410-502-3629; e-mail: levisma@jhmi.edu.
