Abstract
Over the past 2 decades, dramatic improvements in the efficacy of treatments for chronic lymphocytic leukemia have led to progressively higher percentages of clinical complete remissions. A molecular eradication of the leukemia has become not only a desirable, but also an achievable, end point that needs to be evaluated within clinical trials. The assessment of complete remission only at the clinical and morphological level is insufficient, at least for physically fit patients. The detection of minimal residual disease (MRD) in chronic lymphocytic leukemia has become feasible using PCR-based or flow cytometric techniques that reproducibly allow reaching the detection level of less than 1 leukemic cell per 10 000 leukocytes (10−4), the level currently defined as MRD− status. Emerging data indicate that the MRD status during and at the end of treatment is one of the most powerful predictors of progression-free and overall survival. This predictor appears to be independent of clinical response, type or line of therapy, and known biological markers. For these reasons, the time is ripe to test the use of MRD as a surrogate marker of clinical end points and as a real-time marker of efficacy and/or resistance to the administered therapies. In the near future, clinical trials will determine whether MRD assessment can be used for guiding therapy, either to improve quality of responses through consolidation or to prevent relapses through preemptive therapies based on the reappearance of MRD.
Introduction
Handle with care! This warning should appear when dealing with the biological prognostic factors in chronic lymphocytic leukemia (CLL). On one side, it is beyond doubt that studies on prognostic factors have helped to unravel new mechanisms central to the pathogenesis of the disease.1 The best example is probably represented by the analysis of the BCR Ig genes that led to the now well-established concept of antigenic stimulation in the onset and progression of CLL (see manuscript by Chiorazzi). This understanding has recently culminated with the possibility of targeting the signaling pathways originating from the BCR and has rapidly translated into the clinical setting with the introduction of inhibitors currently being tested in clinical trials (see manuscript by Wiestner).
On the other side, after a decade of experimental work, these novel biological prognostic markers have not yet been able to hold the initial promise of providing us with the possibility of predicting for each individual patient the clinical prognosis at the time of initial diagnosis. Indeed, many of these new markers are able to define the clinical outcome at population level with a high predictive value (up to 80% of correlation). However, this leaves a rather wide (approximately 20%) margin of error when assessing these markers to predict prognosis at the level of the individual patient, thereby hampering the possibility of using them effectively in everyday practice.2 A notable exception exists: the presence of deletion of chromosome 17p and/or mutations of the TP53 gene are associated with refractory disease and a very short overall survival.3 Still, no decision can be taken on these markers at the time of initial diagnosis because their association with dismal prognosis depends on the confirmation of clinical progression and they can be acquired during the course of the disease.
A look into the future of prognostication
Ten years have not passed in vain and many of our concepts in the field of prognostication in CLL have evolved thanks to the knowledge that we have accumulated so far, opening new possibilities for the future.
First of all, we have learned that in case of progression, response to therapy is the most important prognostic factor for survival.4 While prognostic factors should help in identifying patients who will eventually require treatment, predictive markers should identify among the progressing patients those who will respond to a given treatment. This would help in selecting the most efficacious therapies for each individual case (tailored therapy), sparing patients from ineffective drugs that would only cause unwanted toxicity and favor the emergence of resistant clones.
Second, some of the novel biological treatments (including immunomodulators, signalling inhibitors, mAbs, and immunotherapeutic approaches) appear to be active irrespective of the presence of negative prognostic features. In addition, such nonchemotherapeutic compounds appear to show relevant clinical efficacy together with milder side effects. If confirmed, these and other novel drugs may open the path to different treatment modalities aimed at more profound remissions with long-term control of the disease, possibly even cure. The possibility to create more efficacious associations with standard chemotherapy without increasing hematologic toxicities and/or to prolong drug administration in consolidation or maintenance strategies may indeed shift the paradigm of therapy in CLL from palliative care to eradication of the disease, at least to a level resulting in a survival benefit.
How can we get there?
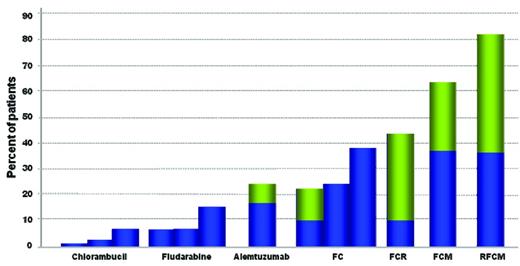
We are now in an era in which the novel immunochemotherapeutic combinations have shown greater efficacy in obtaining progressively higher percentages of clinical complete remissions (CRs; Figure 1) and, in the case of fludarabine/cyclophosphamide/rituximab (FCR), a survival advantage.5 Based on this, the complete eradication of the leukemia becomes an obvious desired end point in the context of clinical trials,6 and the simple assessment of complete remission at the clinical and morphological level will simply not suffice, at least for physically fit patients receiving combined chemoimmunotherapy treatment.
Increasing percentages of CR and MRD− CR after first-line treatment in CLL patients. The results presented are after the administration of: chlorambucil30,50,51 ; fludarabine50–52 (for which only clinical responses are shown); alemtuzumab30 ; the FC combination5,50,52 ; FCR5 ; FCM32 ; RFCM31 (for which MRD− cases as assessed by high-sensitivity methods are shown). Percentages of MRD+ cases or those without MRD evaluation are depicted in blue; MRD− cases are in green.
Increasing percentages of CR and MRD− CR after first-line treatment in CLL patients. The results presented are after the administration of: chlorambucil30,50,51 ; fludarabine50–52 (for which only clinical responses are shown); alemtuzumab30 ; the FC combination5,50,52 ; FCR5 ; FCM32 ; RFCM31 (for which MRD− cases as assessed by high-sensitivity methods are shown). Percentages of MRD+ cases or those without MRD evaluation are depicted in blue; MRD− cases are in green.
It is now clear that many patients achieving a clinical CR by the International Workshop on Chronic Lymphocytic Leukemia guidelines6 will still have minimal residual disease (MRD). Therefore, in studies aimed at maximizing the CR rate, the quality of the CR should be also assessed for the absence of MRD. MRD assessment has been already included in clinical trials of CLL for at least 20 years, starting with less sensitive techniques. Over the past 2 decades, we have witnessed huge improvements in MRD detection that paralleled the dramatic changes in treatment strategies and the increase in the quality of the clinical responses (Figure 1).
Today, although technically one can reach the detection of less than 1 CLL cell in 1 000 000 leukocytes (0.0001% or 10−6), technologies validated in multicenter studies can reliably and reproducibly detect the presence of less than 1 CLL cell in 10 000 leukocytes (0.01% or 10−4), the level currently defined as MRD− status according to international guidelines.6 Therefore, the possibility of measuring MRD effectively is provided only by the use of techniques that allow reaching this detection threshold.
MRD: how to detect it
MRD can be assessed by distinct approaches, either based on PCR or on flow cytometry. For both approaches, different methods exist that allow us to reach strikingly different levels of sensitivity (above or below the threshold of negativity of 10−4). It is now evident that less sensitive and specific techniques (eg, qualitative PCR and 2-color flow cytometry) are not capable of reaching the requested threshold in a large proportion of cases and should not be used to assess the MRD status. That notwithstanding, low-sensitivity techniques are still worth mention, both for historical reasons and to be able to interpret older studies. They have had the merit of demonstrating for the first time that it was possible to improve the definition of the quality of response beyond clinical and morphological assessment, with clinical benefits in terms of improved progression-free survival (PFS) in patients with no evidence of residual lymphoid cells.
PCR-based techniques
Low-sensitivity: consensus PCR.
Every leukemic B-cell clone carries a unique IGHV-IGHD-IGHJ rearrangement that can be amplified by PCR using consensus primers for the IGHV and IGHJ genes at the 5′ and 3′ region of the rearrangement, respectively.7
The use of this PCR reaction will allow, in a qualitative approach, the detection of residual leukemic cells that will be indicated by the presence of a distinct, monoclonal PCR product of the same size as the diagnostic product sample, usually intermixed with a polyclonal background due to the presence of normal B lymphocytes. The ability to visualize the presence of residual disease varies depending on the size of the Ig PCR product and the number of normal B cells present. The sensitivity of the assay can be as high as 1:1000 (10−3 or 0.1%) and is therefore more sensitive than 2-color flow cytometry.8 However, it can be much less sensitive in a regenerative BM and is always less sensitive than quantitative PCR or multicolor flow cytometric methods.9–11
High-sensitivity: clone-specific PCR.
The sensitivity of the PCR can be improved by taking advantage of the uniqueness of the Ig gene rearrangement in each leukemic clone. After sequencing the clonal IGHV-IGHD-IGHJ gene rearrangement at the time of diagnosis, a primer can be designed in the complementary determining region of the heavy chain (VH CDR3), the most variable and “clone-specific” part of each rearrangement. This can be used for a second (“nested”) round of PCR after the first-step consensus PCR. Although this approach is definitely more sensitive (up to 10−6),12,13 it does not allow the quantification of MRD levels, thereby hampering a reliable comparison between samples. For these reasons, today the preferred molecular approach for MRD assessment is real-time quantitative PCR (RQ-PCR), which combines the use of clone-specific sequences with the quantification of the PCR copy numbers and allows us to reach sensitivities in the range of 10−4 to 10−5 (1:10 000/1:100 000 or 0.01%-0.001%).9,12,14
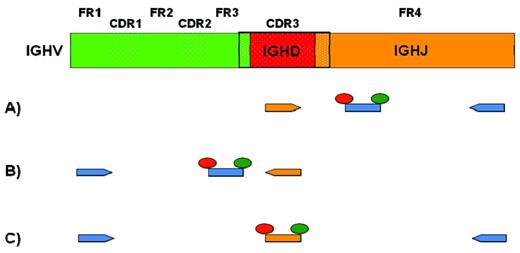
For the RQ-PCR, in addition to a forward and a reverse primer, a probe is required. Different strategies have been proposed (Figure 2).12,14,15 In general, patient-specific primers (allele-specific oligonucleotides) are designed within the VH CDR3 sequence but in opposite directions depending on the approach taken. With the forward patient-specific primer (Figure 2A), the reverse consensus primer will be located in the 3′ end of the IGHJ gene and the probe will be located in between, on a more internal consensus sequence of the IGHJ gene. In the case of a reverse patient-specific primer (Figure 2B), the forward consensus primer will be within the IGHV gene, whereas the probe will be designed in the FR3 portion of the IGHV gene. In either case, this methodology remains labor intensive because it requires the sequencing of each clone-specific IGHV-IGHD-IGHJ rearrangement and the subsequent testing of the primer-probe combination.4,9,10 In addition (Figure 2C), a clone-specific probe can be designed on the VH CDR3 portion of the rearrangement and used with a forward consensus primer on the IGHV gene and a reverse one on the IGHJ gene.16 This approach is more rarely used given the extra cost of the production of a new probe for each patient and the higher probability of competition on polyclonal Igs for the binding of the consensus primers.
PCR approaches for MRD detection. Top row is a schematic representation of the variable domain of the IGH chain derived from the rearrangement of an IGHV (green), an IGHD (red), and an IGHJ (orange) gene. Dotted areas represent complementarity determining regions (CDR1-3). Full areas represent framework regions (FR1-4). One to 3 different approaches for allele-specific oligonucleotide RQ-PCR are represented (see text for details). Consensus primers and probes are depicted in blue. CDR3-specific primers and probes are depicted in orange.
PCR approaches for MRD detection. Top row is a schematic representation of the variable domain of the IGH chain derived from the rearrangement of an IGHV (green), an IGHD (red), and an IGHJ (orange) gene. Dotted areas represent complementarity determining regions (CDR1-3). Full areas represent framework regions (FR1-4). One to 3 different approaches for allele-specific oligonucleotide RQ-PCR are represented (see text for details). Consensus primers and probes are depicted in blue. CDR3-specific primers and probes are depicted in orange.
Moving forward: high-throughput approach.
More recently, another methodology has been proposed that is exploiting a high-throughput IGHV-IGHD-IGHJ sequencing approach.17 Although there is no improvement in terms of sensitivity (approximately 10−5), this technique has the advantage that MRD quantification is obtained using consensus primers without the need for patient customization, therefore in principle making it more generally applicable. Although costs are likely to come down significantly in the near future, this is currently a very expensive approach and interpretation of the results requires significant bioinformatics support. The future will tell if and how such an approach can be successful and reliable compared with its predecessors.
Flow cytometric protocols
CLL cells show a characteristic and unique phenotype, CD19+, CD20dim, CD5, sIGdim that has been exploited since the beginning of the MRD era to distinguish low amounts of leukemic cells reliably within a polyclonal B-lymphocyte background using flow cytometry.18
Low-sensitivity: 2-color flow cytometry.
Initially, a simple approach was used based on the detection of only 2 antigens, CD1919 or CD20,20,21 in combination with CD5. The presence of more than 10% of CD20+CD5+ cells/total lymphocytes or more than 25% of CD19+CD5+ cells/total CD19+ cells in the BM was considered as positive for residual disease. The assessment of the light-chain expression and ratio could also added, albeit again in a 2-color fashion, in combination with CD19.20
This approach is not specific, as indicated by the detection of a high proportion of normal CD5+ B cells after effective treatment.22 In addition, it is not quantitative and is only capable of reaching a sensitivity of 1 leukemic cell in 200 normal cells, far from what is currently needed to define negativity for MRD.
High-sensitivity: 4-color or more flow cytometry.
The assessment of more antigens in combination with CD19 and CD5 has definitely helped to improve the sensitivity of flow cytometric assays and allowed us to reach levels similar to RQ-PCR (10−4-10−5).23 One has to consider that the assessment of the light chain (kappa/lambda) in addition to CD19/CD5 does not improve significantly the test sensitivity, which is inversely correlated with the number of polyclonal B cells present in the sample.
This limitation can be overcome by designing marker combinations directed against the disease-specific phenotype. Such approaches are typically based on the assessment of the expression on the leukemic cells of 2 or more antigens in addition to CD5 and CD19 using 4 or more flow cytometry detectors/colors. Several Ab combinations9,11,24 have been reported and all are able to detect CLL cells when they represent at more than 1:10 000 (0.01% or 10−4) of total leukocytes. This sensitivity is at least 10-fold higher than the methods based on CD19/CD5/κ/λ combinations.
Results from several studies helped in establishing the concept that multicolor (4 or more colors) flow cytometry can reach a sensitivity comparable to that of RQ-PCR,9,10,15,23 with the obvious advantage of being simpler and faster without requiring the design of clone-specific primers. For these reasons, there has been a worldwide effort, coordinated by the European Research Initiative on CLL (ERIC), to develop consensus multicolor flow cytometry panels that could be used reliably in the context of multicenter clinical trials.23
The current standardized panel is a 4-color assay including the following 3 combinations, which are the ones with the lowest interlaboratory variation among several tested: (A) CD5/CD19/CD20/CD38; (B) CD5/CD19/CD81/CD22; and (C) CD5/CD19/CD79b/CD43.
MRD should be detected by at least 2 of the 3 combinations. This assay is equally effective using either peripheral blood or BM regardless of the type of therapy used, with the notable exception of patients treated with Ab-containing regimes in which BM aspirate is necessary to assess MRD in the first 3 months after completion of therapy.23
This international collaborative effort also helped in establishing 2 additional concepts that need to be considered when performing MRD detection by flow cytometry in a diagnostic setting. First, a combination of Abs against CD19 and CD3 is recommended as contamination control and to determine the limit of detection (CD19/CD14/CD3/CD45 in the original 4-color assay). Second, all MRD analysis should be preceded by an initial screening using the CD19/CD5/κ/λ combination because this is able to detect the presence of residual leukemic cells with a positive predictive value of 100% in almost half of the samples proposed for MRD assessment.25 Only the remaining half of the samples should then undergo an extended MRD analysis, thereby avoiding unnecessary work and cost in a great proportion of cases in a typical diagnostic setting.
These 4-color combinations have been used worldwide for MRD detection in the context of clinical trials, but efforts have been made to improve the assay further. Due to the need to test separately 5 individual combinations, this assay can indeed be time-consuming for both the acquisition and the result analysis.
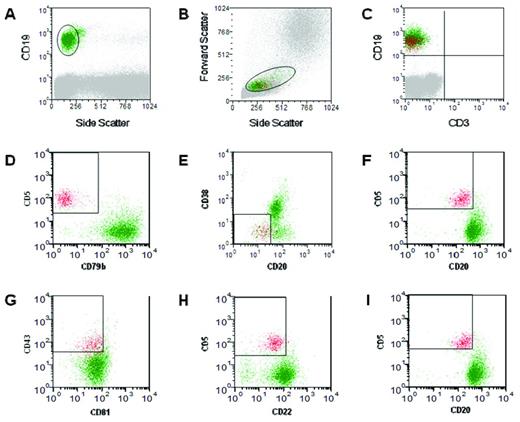
A 6-color assay (Figure 3) has now been tested and validated, again under the coordination of the ERIC,25 allowing the simultaneous exclusion of contaminating events and the distinction of CLL cells from normal B lymphocytes, in only 2 tests (Figure 2) other than light chain restriction: (A) CD19/CD5/CD20/CD3/CD38/CD79b and (B) CD19/CD5/CD20/CD81/CD22/CD43.
MRD analysis using 6-color flow cytometry assay. A representative analysis of a peripheral blood sample is shown, in which the presence of an obvious MRD population can be seen. Analysis was performed after gating cells based on the expression of CD19 (A) and the side/forward scatter profile (B) in both test tubes (see text). Leukemic cells are shown in red in panels C-H. For the first test tube, the CD19/CD3 combination (C) is used to calculate the number of CD3+ events in the B-cell gate and the combination of 3 gates based on CD5, CD20, CD79b, and CD38 expression (D-F) is used for the calculation of events with a CLL-like phenotype. For the second test tube, MRD events are identified through 3 gates based on the expression of CD5, CD20, CD22, CD43, and CD81 (G-I).
MRD analysis using 6-color flow cytometry assay. A representative analysis of a peripheral blood sample is shown, in which the presence of an obvious MRD population can be seen. Analysis was performed after gating cells based on the expression of CD19 (A) and the side/forward scatter profile (B) in both test tubes (see text). Leukemic cells are shown in red in panels C-H. For the first test tube, the CD19/CD3 combination (C) is used to calculate the number of CD3+ events in the B-cell gate and the combination of 3 gates based on CD5, CD20, CD79b, and CD38 expression (D-F) is used for the calculation of events with a CLL-like phenotype. For the second test tube, MRD events are identified through 3 gates based on the expression of CD5, CD20, CD22, CD43, and CD81 (G-I).
Interestingly, this approach has a very good correlation with the 4-color panel, with a good linearity even down to the 0.001% (10−5) level.25 As anticipated, this high sensitivity was reached using fewer reagents and requiring fewer cells and a much simpler analysis approach.
An additional step will be the development of a single test assay (in addition to the initial screening for light-chain assessment) thanks to an 8-color panel that is currently being tested worldwide (www.ericll.org/projects). This includes CD19/CD5/CD20/CD3/CD81/CD79b/CD22/CD43.
Going beyond complete remissions
Even with the use of qualitative methods as 2-color flow cytometry with or without light-chain assessment, it soon became evident that it was possible to increase sensitivity far beyond the clinical and morphological level and thus assess far more accurately and reliably the quality and depth of the response with diverse treatments, including: fludarabine/ prednisone,20 fludarabine/ cyclophosphamide,26 cladribine/ cyclophosphamide27 ; pentostatin/ cyclophosphamide/ rituximab,28 fludarabine/ cyclophosphamide/ rituximab,29 or alemtuzumab alone.30 The use of high-sensitivity techniques has corroborated this evidence, proving that attainment of MRD eradication (< 10−4) is a goal achievable with the most modern immunochemotherapeutic combinations (Figure 1).5,31–36
Is MRD a predictor of clinical outcome?
It became obvious to ask whether it was clinically meaningful to reach MRD− status. Interestingly, even the earlier studies using qualitative assays had suggested that MRD negativity could predict the outcome with longer PFS20,28,29 and overall survival (OS)37 in patients in whom no residual leukemic cells could be detected. More recently, the use of high-sensitivity methods confirmed and further extended this knowledge with more significant differences in survival. This was true in relapsed/refractory patients achieving MRD− status, who showed an improvement in both PFS and OS after alemtuzumab as single-agent therapy.35 More importantly, similar results were also obtained after fludarabine/cyclophosphamide (FC) first-line treatment where increased PFS33,38 and OS38 were associated with achieving < 10−4 MRD. Conversely, the persistence of MRD positivity after autologous stem cell transplantation (alloSCT), although no longer considered a standard treatment in CLL,39 showed a strong correlation with clinical progression.10,11,24,40
Interestingly, it also became evident that the lower the level of MRD (defined as high, > 10−2; intermediate, > 10−4 to < 10−2; low, < 10−4), the better the outcome. Significant differences were seen in PFS at any given point of assessment and for all MRD levels, and also in OS, although only between low and intermediate compared with high MRD levels, probably due to a short follow-up.38
Previous studies in the context of autologous SCT obtained similar results, because the levels of MRD were correlated strongly with the risk of relapse, which was 100% at 5 years if MRD was > 10−4 by flow cytometry and 0% if it was < 10−5 by RQ-PCR.10
MRD is an independent prognostic factor
After demonstrating that MRD negativity does carry prognostic power, a couple of questions remained on the table. Is MRD an independent prognostic marker or is it simply associating with the quality of the clinical response and/or the intensity and efficacy of the treatments applied? Similarly, is it possible that the achievement of an MRD status may be merely a surrogate marker for intrinsic biological features of each leukemic clone that make it more prone to respond particularly well to a treatment?41
Very recently, some answers to these questions have been provided by the results of the German CLL Study Group (GCLLSG) CLL8 trial38 that previously demonstrated the efficacy of the addition of rituximab (R) to fludarabine (F) and cyclophosphamide (C) combination administered as first-line therapy in fit CLL patients requiring treatment.5 Bottcher et al assessed MRD status in both arms of the study (FC vs FCR) and showed convincingly for the first time that MRD negativity after 3 cycles of therapy predicted for longer PFS and OS independently from established biological prognostic markers (eg, IGHV gene mutations and cytogenetic abnormalities such as chromosome 17p deletion). In addition, the investigators reported that MRD prognostic power is also independent from the treatment received. In particular, although patients are more likely to reach low-level MRD status after FCR rather than after FC (63% vs 35%), patients with the same level of MRD in either arm had no significant differences in terms of PFS and OS.
Interestingly, a major finding of this work is that MRD appears to be independent of clinical response, because patients in partial remission (PR) achieving low-level MRD experience a prognosis very similar to those in CR. That notwithstanding, CR is more likely associated with low-level MRD, and similar MRD levels are associated with longer PFS in CR compared with PR patients. This evidence underscores the prognostic relevance of the quality of remission after therapy as defined by both MRD levels and clinical staging.
Because MRD is a strong predictor for clinical outcome, it is now being proposed to the regulatory agencies as a meaningful end point for clinical studies in which it could be used as a surrogate for PFS and OS. This would decrease the need for long follow-up in clinical experimentations before confirming or neglecting the efficacy of an experimental drug.
Can we exploit MRD for guiding therapy?
Based on previous results, one may be tempted to use MRD assessment for guiding therapy. In general, 2 scenarios can be envisaged, but both still need to be tested in clinical trials before attempting any use in the general practice. First, reaching an MRD level of 10−4 appears to be clinically meaningful, suggesting the possibility of aiming for this target rather than for clinical response when applying a treatment, at least in young, fit, progressive patients.42 Second, reaching 10−4 per se does not equal eradication of the leukemic clone, although it is considered “negative” by the current guidelines.6 Reappearance of MRD is observed even in patients who reach this low-level MRD, and this inevitably precedes relapse11,35,38 except in the context of alloSCT. This may open the path to assessing the efficacy of preemptive therapies based on the detection of MRD relapse rather than clinical progression.43
MRD as a target for therapy: a door for consolidation/maintenance
Several studies have already suggested that the quality of the response after chemotherapy can be improved by consolidation, for example, with alemtuzumab, allowing us to achieve MRD− status using low-sensitivity44,45 but also high-sensitivity16 PCR-based assays. As for standard therapies, this achievement has translated into significant improvements in PFS.
Similar results were also obtained in consolidation strategies using rituximab,13,46 which were capable of inducing MRD− status in a proportion of patients in CR who experienced longer response durations compared with patients in CR but MRD+, but also to those who were MRD− but did not receive further treatment.46
Interestingly, in one study, rituximab was administered after consolidation for maintenance to patients who were MRD+ immediately after induction or became MRD+ within 1 year after completion of therapy, again with benefits in terms of prolongation of PFS.46
Altogether, these results provide a background for consolidation/maintenance therapy (eg, with immunomodulatory agents or inhibitors in addition to mAbs). Based on the results of the CLL8 trial showing MRD independence from clinical response,38 this approach could be applied to all responding patients in whom MRD remains detectable or reappears during follow-up regardless of whether they are in PR or CR. However, when considering the experimental use of additional therapy aiming at MRD negativity, one should also consider that increasing or prolonging treatment may convey unnecessary risks such as infections, myelotoxicity, and secondary malignancies, as has been witnessed in the past.16
In addition, when maintenance strategies are planned, an experimentally fair and clinically meaningful end point remains to be established. In this particular setting, PFS appears to be rather meaningless and time to subsequent therapy may be a more reasonable end point.
For all of these reasons, the potential clinical benefit of MRD eradication remains to be demonstrated experimentally and is justified only within clinical trials and for fit patients with progressive disease.6
MRD as a tool for preemptive treatment: is molecular relapse meaningful?
It is clear that the probability of clinical relapse depends not only on the actual tumor load after therapy (ie, the MRD level reached at a given time point), but also on the intrinsic growth kinetics of any given clone. In regard to the latter, useful information has been provided by alloSCT that has demonstrated the relevance of monitoring MRD kinetics during follow-up rather than simply checking the quality of response at the end of a given treatment.43
This body of knowledge helped in creating the concept that MRD negativity is a “moving target” over time. Therefore, the kinetics of MRD rather than a single MRD assessment is more meaningful, because it is the increase of MRD over time, and not only its persistence, that is eventually followed by clinical relapse.
The efficacy of T-cell–replete alloSCT essentially relies on the GVL effect exerted by donor T cells that may lead to MRD negativity several months after transplantation.47 In some cases, low-level MRD can be achieved after immune intervention47 (either immunosuppression tapering or donor lymphocyte infusions) or acute or chronic GVHD.12,48,49 This evidence allowed testing the use of preemptive immune interventions. Infusion of donor lymphocytes was prompted on the basis of unfavorable MRD results and was able to lead to CR in a few cases.49
Although the concept of treating patients at molecular relapse is not yet established in CLL, these results set the need to evaluate systematically preemptive interventions, also in the context of standard therapies. Again, this should be tested only in prospective studies and with all necessary caution regarding the onset of side effects due to clinically unnecessary therapies. With this in mind, the appearance of unfavorable MRD results during follow-up may trigger preemptive administration of novel targeted therapies (eg, Abs, immunomodulatory drugs, novel signaling inhibitors, even immunotherapeutic strategies) that, especially when characterized by a lower toxicity profile, may be also proposed and evaluated in patients who are still likely in clinical CR.
Prospective clinical trials are also needed to clarify whether MRD assessment may guide a premature stop of the planned cycles to reduce toxicity while maintaining the same efficacy. Interestingly, the CLL8 trial showed that low-level MRD was correlated with clinical outcome irrespective of the time when it was reached, although all patients were eventually scheduled to receive all 6 cycles of therapy. Prospective clinical trials need to be designed to test specifically whether a policy of therapy de-escalation is worth being pursued.
A look into the future: ongoing clinical trials
Studies designed along these new concepts are now enrolling patients worldwide. In particular, the GCLLSG is proposing a study (CLLM1) in which physically fit patients with a high risk of early progression after first-line immunochemotherapy (FC, FCR, or BR) are randomized to maintenance therapy with lenalidomide versus placebo. “High risk” is defined on the presence of MRD after responding to first-line therapy as either ≥ 10−2 or ≥ 10−4 to <10−2, in the latter case with the presence of at least one of the following adverse prognostic markers: unmutated IGHV gene status, 17p deletion, or TP53 gene mutation. This trial will also attempt an MRD-guided approach, as the dose of lenalidomide will be increased if and when MRD is still detectable at predefined time points. In addition, in the United Kingdom under the guidance of the National Cancer Research Institute (NCRI) CLL subgroup, the CLARET study, in which patients who have responded to previous chemotherapy and have detectable MRD (> 10−4) will be randomized to receive either consolidation therapy with alemtuzumab for 6 weeks or no therapy, has begun. These and similar studies will help in clarifying the potential benefits of achieving MRD− status in patients with CLL.
Conclusions
Emerging data indicate that MRD status at the end of treatment is one of the most powerful predictors of PFS and OS, and is independent of the clinical response, the type or line of therapy, and biological markers. The time is now ripe to test the use of MRD as a surrogate marker of clinical end points. In particular, the analysis of MRD kinetics should be evaluated as a real-time marker of efficacy and/or resistance to the administered therapies. That being said, MRD status should not be used to guide therapy outside of clinical studies because it is still the subject of ongoing research. The benefits of achieving MRD− status in patients with CLL require further investigation and need to be proven in large, controlled trials. Also important is the definition of the risks of adverse events occurring from unnecessary therapies delivered to patients in good clinical remission, so far the best established prognostic marker we have. Finally, we should consider that MRD will not likely be a goal for every CLL patient; rather, it should be considered based on the fitness status. The use of high-sensitivity tests, and in particular of multicolor flow cytometry, will be critical to the success of this research agenda.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Paolo Ghia, Università Vita-Salute San Raffaele, Via Olgettina 58, 20132 Milano, Italy; Phone: +39-02-2643-4797; Fax: +39-02-2643-4723; e-mail: ghia.paolo@hsr.it.