Abstract
Venous thromboembolism (VTE) is an important cause of preventable morbidity and mortality in medically ill patients. Randomized controlled trials indicate that pharmacologic prophylaxis reduces deep venous thrombosis (relative risk [RR] = 0.46; 95% confidence interval [CI], 0.36-0.59) and pulmonary embolism (RR = 0.49; 95% CI, 0.33-0.72) with a nonsignificant trend toward more bleeding (RR = 1.36; 95% CI, 0.80-2.33]. Low-molecular-weight heparin (LMWH) and unfractionated heparin are equally efficacious in preventing deep venous thrombosis (RR = 0.85; 95% CI, 0.69-1.06) and pulmonary embolism (RR = 1.05; 95% CI, 0.47-2.38), but LMWH is associated with significantly less major bleeding (RR = 0.45; 95% CI, 0.23-0.85). LMWH is favored for VTE prophylaxis in critically ill patients. New VTE and bleeding risk stratification tools offer the potential to improve the risk-benefit ratio for VTE prophylaxis in medically ill patients. Intermittent pneumatic compression devices should be used for VTE prophylaxis in patients with contraindications to pharmacologic prophylaxis. Graduated compression stockings should be used with caution. VTE prevention in medically ill patients using extended-duration VTE prophylaxis and new oral anticoagulants warrant further investigation. VTE prophylaxis prescription and administration rates are suboptimal and warrant multidisciplinary performance improvement strategies.
Introduction
More than 700 000 Americans are hospitalized each year for venous thromboembolism (VTE) and as many as 50 000-100000 die from pulmonary embolism (PE).1 Among survivors, 40% will suffer a recurrent VTE within 10 years and up to 50% will develop postthrombotic syndrome.2,3 Although the risk of VTE is greater among surgical patients, medically ill patients have more episodes of VTE and are less likely to have received VTE prophylaxis.4 Therefore, VTE prevention among the medically ill is an important patient safety and quality of care issue.
Methods
Inclusion criteria
Randomized controlled trials (RCTs) comparing pharmacologic prophylaxis with no pharmacologic prophylaxis in hospitalized medically ill patients were included to evaluate the efficacy of pharmacologic prophylaxis. RCTs of low-molecular-weight heparin (LMWH) versus unfractionated heparin (UFH) in hospitalized medically ill patients were included to evaluate the effectiveness of LMWH and UFH. Clinical outcomes abstracted were deep venous thrombosis (DVT), PE, fatal PE, symptomatic VTE, major bleeding, and all-cause mortality.
Statistical analysis
Individual clinical outcomes reported in RCTs of pharmaceutical prophylaxis versus no prophylaxis and RCTs of LMWH versus UFH were pooled for meta-analysis. The relative risk (RR) and 95% confidence interval (CI) were calculated for each study using a random-effects (Der-Simonian and Laird) model. The absolute risk difference (not shown) was calculated to determine the number needed to treat (NNT) and the number needed to harm (NNH). P < .05 was considered significant for all analyses. STATA Version 12.1 statistical analysis software was used for all analyses.
Which medically ill patients should receive VTE prophylaxis?
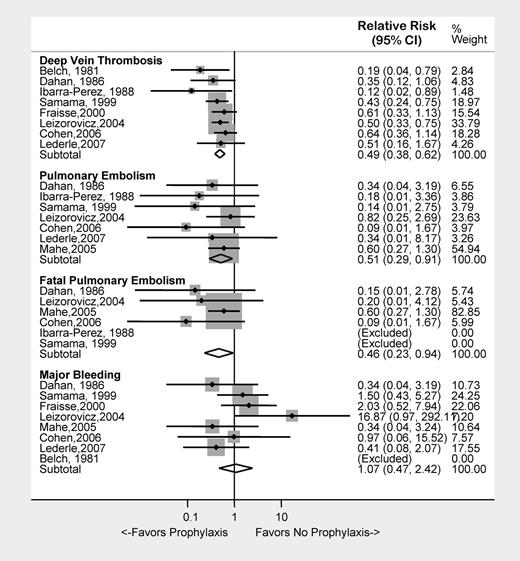
We examined clinical outcomes in 9 studies of pharmacologic prophylaxis in 8617 hospitalized medically ill patients.5–13 VTE prophylaxis with UFH, LMWH, or fondaparinux was associated with a 51% risk reduction for DVT (RR = 0.49; 95% CI, 0.38-0.62) and a 49% risk reduction for PE (RR = 0.51; 95% CI, 0.29-0.91), and a 54% risk reduction in fatal PE (RR = 0.46; 95% CI, 0.23-0.94). There was a trend toward more major bleeding in the active treatment group (RR = 1.07; 95% CI, 0.47-2.42; Figure 1). In terms of absolute numbers, pharmacologic prophylaxis prevented 1 DVT for every 22 patients treated (NNT: 22) and 1 PE for every 181 patients treated (NNT: 181), thus far outweighing the risk of major bleeding (NNH: 707).
Clinical outcomes associated with the use of pharmacologic VTE prophylaxis in medically ill patients.
Clinical outcomes associated with the use of pharmacologic VTE prophylaxis in medically ill patients.
Several limitations of these data warrant discussion. Most DVTs were asymptomatic, a clinical entity of controversial importance. The use of objective radiologic surveillance as a primary end point unavoidably reduced the number of symptomatic VTEs, thereby reducing the utility of this study design for determining symptomatic event rates. We excluded the largest study of pharmacologic VTE prophylaxis due to incomplete information on the timing of pulmonary embolism (in- versus out-patient).14 Therefore, the net clinical benefit of pharmacologic VTE prophylaxis in medically ill patients should be interpreted cautiously.
The recent American College of Physicians (ACP) and American College of Chest Physicians (ACCP) VTE prevention guidelines have emphasized the importance of risk stratification of medically ill patients before prescription of VTE prophylaxis.15,16 A large number of clinical characteristics have been identified as risk factors for VTE among medically ill patients, including age, previous VTE, thrombophilia, cancer, immobility, New York Heart Association (NYHA) class III/IV congestive heart failure, respiratory failure, infections, inflammatory/rheumatologic disease, and stroke. Several different risk assessment models have been proposed.17–19
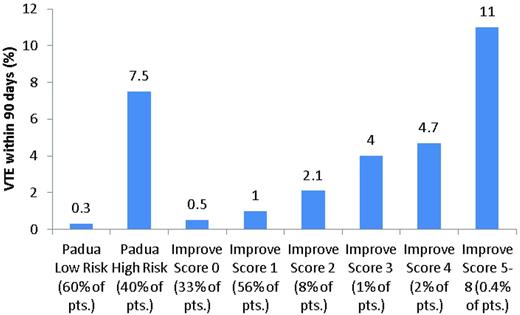
Barbar et al used a modification of the Kucher score, the Padua VTE risk model, to assess medical inpatients in a prospective cohort study conducted in an Italian hospital over a 2-year period (Table 1). Patients with 4 or more points were considered to be at high risk for developing VTE. Of 1180 patients, 469 were judged to be at high risk and 711 to be at low risk for developing VTE. Pharmacologic prophylaxis was administered to 186 high-risk patients (39.7%) and 52 low-risk patients (7.3%). Within 90 days of discharge, VTE developed in 37 patients (3.1%), including 35 high-risk patients (7.5%) and 2 low-risk patients (0.3%; Figure 2). Four high-risk patients who received thromboprophylaxis (2.2%) and 31 who did not (11.8%) developed VTE (hazard ratio [HR] = 32; 95% CI, 4.1-251). Major bleeding developed in 3 high-risk patients who received thromboprophylaxis (1.6%) and in 1 low-risk patient (1.9%).18
Padua VTE risk model18
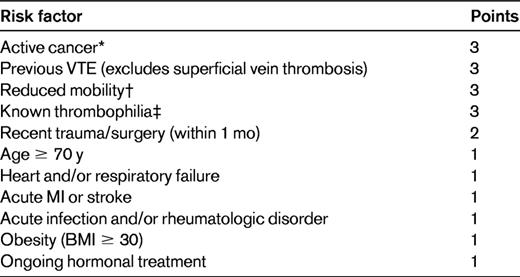
Low VTE risk is 0-3 points; high VTE risk is ≥ 4 points.
BMI indicates body mass index; and MI, myocardial infarction.
*Patients with local or distant metastases or those who received chemo- or radiotherapy within the last 6 mo.
†Patients with bathroom privileges for at least 3 days.
‡Antithrombin, protein C, protein S, factor V Leiden, prothrombin gene mutation, antiphospholipid syndrome.
Cumulative incidence of VTE at 90 days in different risk categories of the Padua and IMPROVE VTE risk scores.
Cumulative incidence of VTE at 90 days in different risk categories of the Padua and IMPROVE VTE risk scores.
Spyropoulos et al developed a VTE risk assessment model based upon data collected as part of the IMPROVE registry (Table 2). Among 15 156 participants, 143 (1%) developed VTE within 3 months. Factors present on admission that were strongly associated with VTE included previous VTE, known thrombophilia, cancer, and age > 60 years. More than 13 000 patients (89%) were classified as low risk (IMPROVE VTE risk score 0-1) and had a 3-month risk of VTE of less than 1%. The 3-month risk of VTE increased with increasing IMPROVE VTE risk score (Figure 2).19
IMPROVE VTE risk score19

Low VTE risk is 0-1 points; high VTE risk is 2 or more points.
*Antithrombin, protein C, protein S, factor V Leiden, prothrombin gene mutation, or antiphospholipid syndrome.
Although VTE prophylaxis generally appears to be associated with a net benefit for medically ill patients, these VTE risk scores offer the potential to target VTE prophylaxis to the patients at greatest risk, whereby minimizing the risk of exposing low-risk patients to potentially preventable harm from prophylaxis-associated bleeding. An important task for researchers is to validate these risk scores in different patient populations to confirm their reproducibility before their widespread adoption into clinical use. If validated, these risk scores, when paired with a bleeding risk score, will allow clinicians to target VTE prophylaxis to the medically ill patients most likely to benefit from its use. Until these data are available, we will continue to use inclusion and exclusion criteria from RCTs of VTE prophylaxis in the medically ill, such as the PREVENT and MEDENOX studies, to guide VTE prophylaxis decision making.9,11
Which medically ill patients are at greatest risk for bleeding with pharmacologic VTE prophylaxis?
Although pharmacologic prophylaxis is associated with a small risk of major bleeding (< 1%) in most patients, there are a substantial number of medically ill patients for whom anticoagulant prophylaxis is inappropriate.20 Identification of these patients is important because compliance with mechanical prophylaxis is suboptimal and the risks and benefits of mechanical prophylaxis may not be favorable in medical patients.21–23 To address this knowledge deficit, Decousus et al examined bleeding complications in the IMPROVE study population. Eighty-three patients (1.2%) suffered major bleeding and 12 had fatal bleeds (0.1%) within 14 days of admission. Factors associated with an increased risk of bleeding are displayed in Table 3. Patients with less than 7 points (n = 8476, 90.3% of the population) had a major bleed incidence of 0.4%, whereas patients with ≥ 7 points (n = 912, 9.7% of the population) had a major bleed incidence of 4.1%.24 Although it must be validated in other patient populations, the IMPROVE bleeding risk assessment score represents an important step toward defining criteria with which the bleeding risk of medically ill patients can be quantitatively assessed to allow for a tailored approach to VTE prophylaxis.
Bleeding risk factors in medically ill patients24

INR indicates international normalized ratio; GFR, glomerular filtration rate; and CCU indicates coronary care unit.
LMWH versus UFH for VTE prophylaxis in medically ill patients
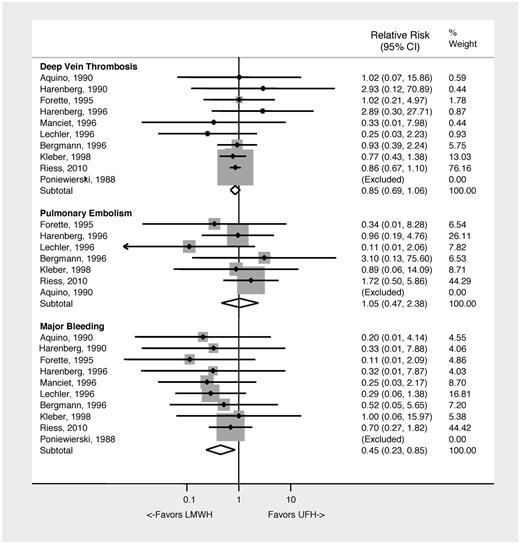
LMWH and UFH have been compared in 10 randomized clinical trials between 1988 and 2010 in 7760 patients.25–34 A meta-analysis of these studies demonstrates no difference in prevention of DVT, PE, or all-cause mortality. LMWH was associated with a 55% relative risk reduction in major bleeding compared with UFH (RR = 0.45; 95% CI, 0.23-0.85]; NNH: 196) (Figure 3). Limitations of this analysis include differences in outcome assessment, subject populations, and anticoagulant regimen between studies. Despite these limitations, the data indicate that UFH or LMWH both prevent VTE, but the use of LMWH appears to be associated with less major bleeding. Table 4 summarizes the recommendations for VTE prophylaxis in medically ill patients.
Clinical outcomes associated with the use of LMWH versus UFH VTE prophylaxis in medically ill patients.
Clinical outcomes associated with the use of LMWH versus UFH VTE prophylaxis in medically ill patients.
Mechanical prophylaxis in the medically ill patient
A substantial proportion of medically ill patients (10%) have contraindications to pharmacologic VTE prophylaxis.35 In these patients, mechanical prophylaxis provides an important alternative to anticoagulant prophylaxis. Graduated compression stockings (GCS) have been examined in only 3 studies of nonsurgical patients, 2 performed in stroke patients and 1 in patients with acute myocardial infarction.23,36,37 Kierkegaard et al used GCS in 80 patients with acute MI, and found that GCS were associated with a significant reduction in DVT; however, this study has been criticized due to its use of an antiquated imaging technique, 125I-fibrinogen uptake.37 Muir et al randomized 98 stroke patients in a 2:1 fashion to GCS stockings or usual care. GCS were associated with a 57% odds reduction (OR = 0.43; 95% CI, 0.14-1.36) in DVT detected on color-flow Doppler ultrasound at 7 days after stroke. No reduction in proximal DVT was noted.36 The CLOTS trial 1 randomized 2518 immobile patients admitted within 1 week of stroke to thigh-length GCS plus routine care or routine care alone. GCS were placed immediately upon randomization and worn day and night until patients were fully mobile or they were discharged from the hospital. Treatment adherence was 79.4% at 14 days and 73% at 30 days. Bilateral compression duplex ultrasound was performed at 7-10 days and 25-30 days postrandomization. The frequency of proximal DVT was similar in the GCS and routine care groups (10% vs 10.5%; 95% CI, −1.9-2.9). No difference in proximal or distal DVT (symptomatic or asymptomatic) or PE was noted. In contrast, GCS recipients were 4.2-fold (95% CI, 2.4-7.3) more likely to develop skin breakdown, skin ulcers, or skin necrosis than routine care patients. This study indicates that GCS do not reduce VTE but are associated with harm.23 Although this study was conducted exclusively in stroke patients, it indicates that intermittent pneumatic compression devices (IPCDs) should be preferentially used for mechanical prophylaxis in medically ill patients. If GCS are used for VTE prophylaxis, they should be used with caution. Randomized trials demonstrating the effectiveness of IPCDs in medically ill patients are warranted.
VTE prophylaxis in critically ill patients
Critically ill patients are at substantial risk for VTE. A prospective study of medical-surgical intensive care unit (ICU) patients found that 2.7% (95% CI: 1.1-5.5) had DVT on admission and 9.6% (95%CI: 6.3-13.8) developed DVT during their ICU stay despite universal prophylaxis. Risk factors for DVT included a personal or family history of VTE (HR: 4.0; 95% CI, 1.5-10.3), end-stage renal failure (HR 3.7; 95% CI, 1.2-11.1), platelet transfusion (HR: 3.2; 95% CI, 1.2-8.4), and vasopressor use (HR: 2.8; 95% CI, 1.1-7.2).38 The PROTECT study randomized 3764 ICU patients (75% medical admissions) to dalteparin 5000 units daily or UFH 5000 units twice daily.39 Within 2 days of admission and then twice weekly until discharge, proximal leg duplex ultrasound was performed. Proximal DVT was identified in 109 UFH patients (5.8%) and 96 dalteparin recipients (5.1%; HR = 0.92; 95% CI, 0.68-1.23). PE was diagnosed in 43 UFH patients (2.3%) and 24 dalteparin patients (1.3%; HR = 0.51; 95% CI, 0.3-0.88). Major bleeding developed in 105 UFH patients (5.6%) and 103 dalteparin patients (5.5%; HR = 1.00; 95% CI, 0.75-1.34). Heparin-induced thrombocytopenia was confirmed in 12 UFH patients (0.6%) and 5 dalteparin patients (0.3%; HR = 0.47; 95% CI, 0.16-1.35).39 These results indicate that once-daily dalteparin is as efficacious as twice-daily UFH in the prevention of DVT and is associated with a similar risk of major bleeding. Although there are no data, IPCDs should be used in medical ICU patients at increased risk of bleeding.
Extended duration prophylaxis for medically ill patients
The risk of VTE continues well beyond the hospital stay. Spencer et al noted that 74% of VTEs occur among outpatients who had been hospitalized recently for an acute medical illness or surgery.4 Extended-duration VTE prophylaxis has been demonstrated previously to be beneficial in patients after hip arthroplasty and cancer surgery. Hull et al conducted the only randomized, double-blind, placebo-controlled study of extended-duration VTE prophylaxis in medically ill patients, the EXCLAIM study.40 After 10 ± 4 days of open-label enoxaparin, medically ill patients 40 years old or older with reduced mobility were randomized to enoxaparin 40 mg daily or placebo for 28 ± 4 days.
Extended-duration enoxaparin was associated with a 1.5% absolute risk reduction of VTE (2.5% vs 4%; 95% CI, −2.5% to −0.5%). Proximal DVT (2.4% vs 3.9%, risk difference [RD] = −1.5%; 95% CI, −2.6 to −0.4) and symptomatic VTE (0.2% vs 1.2%, RD = −1%; 95% CI, −1.5%-0.4%) were also reduced, but no difference in PE was noted (0.1% vs 0.3%, RD = −0.2%; 95% CI, −0.5%-0.05). Major bleeding was increased (0.8% vs 0.3%; 95% CI, 0.1-0.9%). All-cause mortality was similar (2.1% vs 2.2%; HR = 0.93; 95% CI, 0.7-1.3) In subgroup analyses, the reduction in VTE seen with extended duration prophylaxis was restricted to patients age 75 years or older (OR = 0.35; 95% CI, 0.2-0.62), women (OR = 0.40; 95% CI, 0.24-0.65), and immobilized patients (OR = 0.51; 95% CI, 0.31-0.83).40 The EXCLAIM study demonstrates that extended VTE prophylaxis reduces symptomatic VTE in medically ill patients, but the benefits appear to be limited to immobilized patients, patients age 75 years and older, and women.
New oral anticoagulants for VTE prophylaxis in medically ill patients
The ADOPT study compared the oral factor Xa inhibitor apixaban 2.5 mg twice daily to enoxaparin 40 mg once daily in a placebo-controlled, double-blind, double-dummy study of 6528 medically ill patients age 40 years or older with congestive heart failure or respiratory failure or other medical illness in conjunction with one additional VTE risk factor.41 Apixaban or placebo was given for 30 days and enoxaparin or placebo was given for 6-14 days. Seventy patients receiving enoxaparin (3.1%) and 60 receiving apixaban (2.7%) developed VTE (RR = 0.87; 95% CI, 0.6-1.2). Major bleeding developed in 15 apixaban patients (0.47%) and 6 enoxaparin patients (0.19%), a 2.6-fold increased risk among apixaban recipients (95% CI, 1-7.2). Only 26% of ADOPT participants had severely restricted mobility.41 These results indicate that extended prophylaxis with apixaban was not superior to a 6- to 14-day course of enoxaparin. It remains to be seen if a higher risk subgroup of medical patients would benefit from extended prophylaxis with one of the new oral anticoagulants.
Does VTE prophylaxis reduce mortality in medically ill patients?
PE is an important cause of potentially preventable mortality in hospitalized patients. UFH for VTE prophylaxis was previously found to be associated with a significant reduction in mortality (10.9%-7.8%) in an open randomized trial.42 The LIFENOX study was a placebo-controlled RCT of enoxaparin 40 mg daily plus elastic stockings versus placebo and elastic stockings for 10 ± 4 days in 8307 medically ill patients 40 years of age or older hospitalized for acute congestive heart failure, severe systemic infection with 1 other VTE risk factor or cancer.43 Clinical suspicion of VTE was similar between the enoxaparin (0.5%) and placebo (0.7%) groups. Fatal PEs occurred in 1 patient in each arm. All-cause mortality at 30 days was 4.8% among enoxaparin recipients and 4.7% among placebo recipients (RR = 1.0; 95% CI, 0.8-1.2). Major bleeding occurred in 0.4% of enoxaparin patients and 0.3% of placebo patients (RR = 1.4; 95% CI, 0.7-3.1).43 These results indicate that VTE prophylaxis does not reduce all-cause mortality.
Performance improvement on the front lines: how can we increase delivery of VTE prophylaxis to patients?
International surveys have documented that less than half of medically ill patients receive risk-appropriate VTE prophylaxis.35,44 Although provider education is important, education alone is insufficient to effect durable VTE prophylaxis practice change.45 Similarly, “top-down” regulatory approaches such as the Centers for Medicare and Medicaid Services orthopedic surgery “Never Events” ruling target too few patients in an unfocused manner and are fraught with unintended consequences.46 In contrast, electronic alerts, computerized clinical decision support-enabled VTE “smart order sets,” inpatient VTE prophylaxis consultative services, and paper-based VTE risk stratification tools have been demonstrated to increase the rate of risk-appropriate VTE prophylaxis significantly.17,47–50 In light of the wide variety of resources available to improve VTE prevention, hospitals should tailor their strategies to suit their capabilities and budget.
Recent evidence indicates that orders for risk-appropriate VTE prophylaxis do not ensure its administration. Our investigative group and others have noted that up to 12% of doses of pharmacologic VTE prophylaxis are omitted (Fanikos et al51 and Shermock et al, unpublished data.) Patient refusal is the primary reason for missed doses of VTE prophylaxis (up to 59% of missed doses). Our group has noted that a subset of patients (approximately 20%) account for the majority (80%) of missed doses, and provider-patient interactions may contribute to this omission of care. (Shermock et al, unpublished data, and Elder et al, unpublished data). These data underscore the importance of monitoring prophylaxis administration rather than ordering and developing strategies to optimize administered prophylaxis rates.
Disclosures
Conflict-of-interest disclosure: M.B.S. has received research funding from Bristol-Myers Squibb; has consulted for Chason, Rosner, Leary & Marshall LLC, Anderson Daniel LLP, Janssen HealthCare, Daiichi-Sankyo, Eisai, Sanofi-Aventis, and Sands Anderson; and has received honoraria from Sanofi-Aventis and Ortho-McNeil. B.D.L. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Michael B. Streiff, MD, FACP, Associate Professor of Medicine, Division of Hematology, Department of Medicine and Associate Faculty, Armstrong Institute of Patient Safety and Quality Care, Johns Hopkins University School of Medicine, 1830 E Monument St, Ste 7300, Baltimore, MD 21205; Phone: 410-614-0727; Fax: 410-614-8601; e-mail: mstreif@jhmi.edu.