Abstract
The regulation of VWF multimer size is essential in preventing spontaneous microvascular platelet clumping, a central pathophysiologic finding in thrombotic thrombocytopenic purpura (TTP). In the majority of TTP patients, ADAMTS13, the principal regulator of VWF size, is severely deficient. Today, 2 forms of severe ADAMTS13 deficiency are recognized. The acquired form is caused by circulating autoantibodies inhibiting ADAMTS13 activity or increasing ADAMTS13 clearance. Pathogenic anti-ADAMTS13 Abs are mainly of the IgG class, predominantly of subclass IgG4, and inhibitory Abs recognize a defined epitope in the ADAMTS13 spacer domain. The reasons underlying the failure to maintain immunologic tolerance to ADAMTS13, however, are still poorly understood. Constitutional ADAMTS13 deficiency leading to hereditary TTP, also known as Upshaw-Schulman syndrome, is the result of homozygous or compound heterozygous ADAMTS13 gene mutations.
Introduction
Thrombotic thrombocytopenic purpura (TTP) became recognized in 1947, when Singer et al reviewed all 12 cases known at the time, including Moschcowitz's patient, for similarities and differences from other forms of thrombocytopenic purpura.1 The name was ingenious because it not only described the histopathologic finding of generalized arteriolar and capillary platelet thrombosis, but also suggested the most likely pathophysiologic mechanism underlying thrombocytopenia: the consumption of platelets by the formation of innumerable platelet thrombi. The investigators concluded that an appropriate study on the pathogenesis of TTP would only become possible with appropriate disease recognition ante mortem. In the late 1970s, the first reports of successful treatment of acute TTP episodes with plasma exchange or plasma infusions emerged. Among these early reports was the excellent description by Jefferson D. Upshaw of the clinical course of a female patient who presented with recurrent microangiopathic hemolysis and thrombocytopenia, which were reverted when the patient received plasma-containing blood products.2 Upshaw concluded that his patient was similar to a patient described by Schulman et al almost 20 years previously,3 and that both suffered from a congenital deficiency of a plasma factor.
The search for the pathogenesis underlying TTP gained momentum when Moake et al observed the presence of unusually large VWF multimers in the plasma of 4 patients,4 including those of Schulman et al3 (as patient B) and Upshaw2 (as patient D), with a chronic relapsing course of TTP in remission. Moake et al hypothesized that the unusually large VWF, similar in size to VWF multimers in the supernatant media of endothelial cell cultures, were the consequence of a lacking “depolymerase” and accountable for in vivo platelet clumping in the microvasculature observed in TTP.4 The identification of the peptide bond cleaved during physiologic processing of VWF (Tyr1605-Met1606 in the VWF A2 domain) paved the way for the identification of the VWF-cleaving protease by Furlan et al5 and Tsai.6 In 1997, the first link between a chronic relapsing course of TTP, the presence of unusually large VWF multimers in plasma in remission, and missing VWF-cleaving protease activity was established.7 Shortly thereafter, a severe VWF-cleaving protease deficiency caused by circulating IgG autoantibodies inhibiting the protease was found in the majority of adult patients suffering from acquired TTP.8,9 During the ensuing years, the VWF-cleaving protease was purified from plasma and subjected to N-terminal amino acid sequence analysis,10,11 mapped to chromosome 9q34, cloned, and identified as the 13th member of the ADAMTS (a disintegrin and metalloprotease with thrombospondin repeats) family of metalloproteases.12–14 Patients with hereditary TTP were found to carry ADAMTS13 mutations.12
Today, 2 forms of severe ADAMTS13 deficiency (< 5% of the normal amount) are recognized: an acquired form and a congenital form, both leading to sustained VWF-dependent accumulation of platelets in small vessels that eventually results in microvascular thrombosis and TTP (Figure 1).
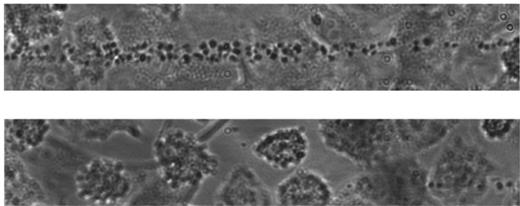
Pathogenesis of TTP. Shown are endothelial cell cultures stimulated to release VWF multimers perfused with fluorescence-labeled platelets suspended in a plasma with a severe ADAMTS13 deficiency of < 5% of the normal (top panel) or in a plasma with normal ADAMTS13 activity (bottom panel). Experiments were performed by Dr R. Fröhlich-Zahnd in the laboratory of Prof J.-F. Dong (at the time with the Department of Medicine, Baylor College of Medicine, Houston, TX, and now at the Puget Sound Blood Center, Seattle, WA).47,48
Pathogenesis of TTP. Shown are endothelial cell cultures stimulated to release VWF multimers perfused with fluorescence-labeled platelets suspended in a plasma with a severe ADAMTS13 deficiency of < 5% of the normal (top panel) or in a plasma with normal ADAMTS13 activity (bottom panel). Experiments were performed by Dr R. Fröhlich-Zahnd in the laboratory of Prof J.-F. Dong (at the time with the Department of Medicine, Baylor College of Medicine, Houston, TX, and now at the Puget Sound Blood Center, Seattle, WA).47,48
Acquired ADAMTS13 deficiency
Circulating anti-ADAMTS13 Abs are found in plasma of nearly all (94%-97%) patients suffering from idiopathic TTP and severe acquired ADAMTS13 deficiency. In the majority of cases, these Abs inhibit ADAMTS13 activity, but 10%-15% of patients have noninhibitory Abs, and severe ADAMTS13 activity is thought to be the result of an increased Ab-mediated clearance. The Abs are mainly of the IgG isotype, predominantly of subclass IgG4, followed by IgG1, IgG2, and IgG3.15 High levels of IgG4 were found in relapsed cases of TTP and were associated with an increased risk of relapse,15 whereas the presence of IgA and/or IgG1 at presentation was associated with adverse outcome in a small number of patients.15,16
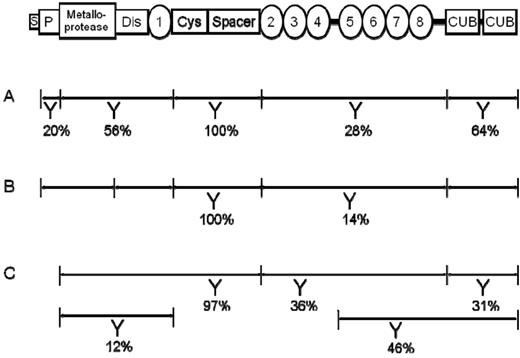
ADAMTS13 Abs recognizing a primary epitope on the ADAMTS13 spacer domain are present in 97%-100% of patients with severe Ab-mediated ADAMTS13 deficiency, and up to 64% of these patients also have Abs that recognize epitopes in other ADAMTS13 domains (Figure 2).17–19 Fine-mapping of the ADAMTS13 spacer domain epitope identified the amino acids Arg568, Phe592, Arg660, Tyr661, and Tyr665 as the primary antigenic target of inhibitory ADAMTS13 Abs.20–22 As yet, the epitopes of ADAMTS13 Abs binding to other ADAMTS13 domains have not been identified.
Schematic protein domain structure of ADAMTS13 and localization of anti-ADAMTS13 autoantibody epitopes. The ADAMTS13 protein consists of 1427 amino acid residues and the domain structure includes a signal peptide (S) and a pro-peptide (P), a metalloprotease, a disintegrin (Dis), a cysteine-rich (Cys), a spacer, and 2 CUB domains, as well as 8 thrombospondin type 1 repeats (1-8) (top row). Bottom rows (A-C): Lines below ADAMTS13 depict the ADAMTS13 fragments used for epitope mapping of anti-ADAMTS13 Abs. Y indicates that Abs directed against the respective ADAMTS13 fragment were found in a certain proportion of patients investigated (percentage indicated). In all 3 studies (A-C), only patients suffering from acute TTP with severe acquired ADAMTS13 deficiency were investigated. Row A: Klaus et al investigated 25 patients using E coli–derived recombinant ADAMTS13 fragments.17 Row B: Luken et al performed epitope mapping in 7 patients using full-length ADAMTS13 and ADAMTS13 fragments produced in insect cells.18 Row C: Zheng et al performed epitope mapping on 67 patient samples using rADAMTS13 and rADAMTS13 fragments produced in mammalian cell lines.19
Schematic protein domain structure of ADAMTS13 and localization of anti-ADAMTS13 autoantibody epitopes. The ADAMTS13 protein consists of 1427 amino acid residues and the domain structure includes a signal peptide (S) and a pro-peptide (P), a metalloprotease, a disintegrin (Dis), a cysteine-rich (Cys), a spacer, and 2 CUB domains, as well as 8 thrombospondin type 1 repeats (1-8) (top row). Bottom rows (A-C): Lines below ADAMTS13 depict the ADAMTS13 fragments used for epitope mapping of anti-ADAMTS13 Abs. Y indicates that Abs directed against the respective ADAMTS13 fragment were found in a certain proportion of patients investigated (percentage indicated). In all 3 studies (A-C), only patients suffering from acute TTP with severe acquired ADAMTS13 deficiency were investigated. Row A: Klaus et al investigated 25 patients using E coli–derived recombinant ADAMTS13 fragments.17 Row B: Luken et al performed epitope mapping in 7 patients using full-length ADAMTS13 and ADAMTS13 fragments produced in insect cells.18 Row C: Zheng et al performed epitope mapping on 67 patient samples using rADAMTS13 and rADAMTS13 fragments produced in mammalian cell lines.19
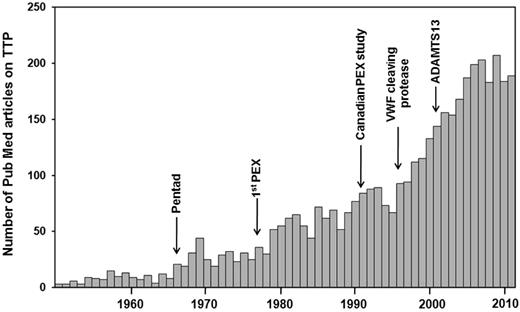
The mechanisms leading to loss of self-tolerance and induction of autoimmunity in acquired TTP are still poorly understood (for a detailed review, see Pos et al23 ). As in other autoimmune diseases, microbial agents have been implicated in the onset of acquired TTP. Given the large variety of microbial agents suspected to play a pathophysiologic role in TTP, it is difficult to assess whether these are indeed causative triggers or whether the associations described represent a publication bias due to the lasting interest in this rare disease (annual incidence, 1.72 cases/million24 ) with a steadily increasing number of annual publications (Figure 3). In this review, we emphasize 3 cases of proven enterohemorrhagic Escherichia coli infection and typical hemolytic uremic syndrome (D+HUS), in which ADAMTS13 activity is usually normal or only mildly reduced. In a study of 29 children with D+HUS, one was found to have a severe deficiency of ADAMTS13 activity, which normalized in remission.25 During the German E coli O104:H4 outbreak in 2011, plasma samples of 6 patients with severe clinical presentation, including neurologic symptoms, were referred to our laboratory for ADAMTS13 activity determination. Two of these 6 patients had a severe acquired ADAMTS13 deficiency due to inhibitory autoantibodies of IgG isotype. One of the 2 patients has since experienced a relapse of thrombotic microangiopathy, displaying again a severe acquired ADAMTS13 deficiency and inhibitor (Prof B. Pötzsch, Experimental Hematology and Transfusion Medicine, University of Bonn, Germany; personal communication). Whether these 3 D+HUS cases were unfortunate to suffer from 2 rare diseases (acquired TTP with severe ADAMTS13 deficiency and D+HUS) at the same time, or if enterohemorrhagic E coli in certain circumstances can trigger the formation of anti-ADAMTS13 autoantibodies, or if Shiga toxin, in analogy to the situation in ADAMTS13 knockout mice,26 was a trigger in patients with so far asymptomatic autoantibody-mediated ADAMTS13 deficiency remains to be elucidated.
Investigator fascination with TTP. Shown are publications retrieved from PubMed using the search term “thrombotic thrombocytopenic purpura.” Given the annual incidence of TTP (idiopathic TTP, 4.5 cases/million; idiopathic TTP with severe ADAMTS13 deficiency, 1.72 cases/million24 ), there seem to exist almost more publications than cases. PEX indicates plasma exchange. The pentad of clinical signs of TTP was described by Amorosi and Ultmann,51 the Canadian plasma exchange study was reported by Rock et al.52
Investigator fascination with TTP. Shown are publications retrieved from PubMed using the search term “thrombotic thrombocytopenic purpura.” Given the annual incidence of TTP (idiopathic TTP, 4.5 cases/million; idiopathic TTP with severe ADAMTS13 deficiency, 1.72 cases/million24 ), there seem to exist almost more publications than cases. PEX indicates plasma exchange. The pentad of clinical signs of TTP was described by Amorosi and Ultmann,51 the Canadian plasma exchange study was reported by Rock et al.52
Evidence for a genetic predisposition to developing pathogenic ADAMTS13 Abs comes from the following 3 observations. First, the MHC class II allele HLA DRB1*11 was found to be overrepresented in patients suffering from acute TTP with severe acquired ADAMTS13 deficiency in France, the United Kingdom, and Germany.27–29 Conversely, the HLA DRB1*04 allele frequency was higher in healthy controls in all 3 populations, hinting at a protective effect of this MHC class II allele. Second, the report on identical twin sisters not carrying the HLA DRB1*11 allele who developed acute acquired TTP with severe ADAMTS13 deficiency due to inhibitory autoantibodies 14 months apart30 suggests additional, thus far unrecognized genetic risk factors. Third, a considerable number (11% and 9.6%, respectively) of heterozygous carriers of ADAMTS13 mutations were found among patients diagnosed with acute acquired TTP and severe ADAMTS13 deficiency.31,32 Seven mutation carriers of these 2 reports had ADAMTS13 Abs of the IgG isotype, which were shown in 6 patients to be functional ADAMTS13 inhibitors. Given the low prevalence of ADAMTS13 mutations in the population, the number of heterozygous mutation carriers among TTP patients with severe acquired ADAMTS13 deficiency is remarkable and suggests a role of heterozygous sequence variants either in the induction of autoantibodies or in facilitating an ADAMTS13 decrease below a threshold relevant for the induction of TTP.
A role of T cells in the deregulated immune response in acquired TTP is likely, but has not yet been formally investigated. In addition to the MHC class II link, somatic hypermutation observed in ADAMTS13 mAbs derived from peripheral B cells and from switched memory B cells isolated from the spleen suggests a role for helper T cells during ADAMTS13 autoantibody formation.18,33 Further evidence comes from the clinical observation during plasma exchange therapy that 30%-50% of TTP patients with severe acquired ADAMTS13 deficiency experience a clinical exacerbation that is often associated with an increase of ADAMTS13 Ab and functional inhibitor titers, a phenomenon apparently not observed in patients treated with cyclosporine A, a T-cell immunosuppressant.34
Congenital ADAMTS13 deficiency and hereditary TTP
Hereditary TTP (Upshaw-Schulman syndrome [USS]2,3 ) due to severe congenital ADAMTS13 deficiency is the result of compound heterozygous or homozygous ADAMTS13 gene mutations. More than 140 different ADAMTS13 mutations, which include missense mutations (approximately 60%), small deletions and insertions (approximately 20%), as well as nonsense and splice site mutations, have been identified in affected patients thus far. There is a considerable genetic heterogeneity, with the majority of mutations confined to single families; patients with homozygous mutations are found mainly in families with consanguinity. Two mutations stand out: the single base insertion 4143insA in exon 29 and the missense mutation Arg1060Trp in exon 24 have both been observed in several unrelated families over a wide geographic area. While the single base insertion 4143insA seems to cluster around the Baltic sea in Scandinavia and Moravia,35 the Arg1060Trp mutation has been observed in the United States and in many countries across Europe.32,36
Worldwide, more than 150 patients diagnosed with USS are alive. Available published data seem to confirm earlier findings of an age-dependent clustering of the first TTP attack, with half of these patients presenting with a first acute TTP bout within their first years of life (early onset). The other half seem to remain asymptomatic into early adulthood and to suffer from a first acute attack between 20 and 40 years of age, and a few patients are even asymptomatic until they are > 60 years of age (late onset).37,38 Regardless of the age at disease onset, once a first TTP bout has occurred, USS patients often present with a chronic relapsing course, recurrences of acute episodes often being triggered by events such as pregnancy or heavy alcohol intake.36,39 In addition to age at onset, the clinical presentation in USS patients is variable and may even differ in families and in unrelated patients bearing the same 2 ADAMTS13 mutations. However, patients with neurologic involvement also tend to present with neurologic symptoms during subsequent episodes, whereas others may never present with neurologic findings but suffer from severe renal impairment instead. For this reason, establishing a link between the ADAMTS13 genotype and the clinical phenotype in USS is difficult. It was reported recently that residual ADAMTS13 activity in the range of 0.5%-6.8%, as determined by a 73–amino acid VWF peptide assay, influenced the age of disease onset and episode frequency.38 The prevalent ADAMTS13 mutation Arg1060Trp is particularly frequent in USS patients presenting with a first acute TTP episode during a first pregnancy.32,36 Moreover, the clinical phenotype may also be influenced by defects in other genes, as reported in an Italian USS family in which one of the affected siblings with predominant renal involvement had a complement factor H mutation in addition to her 2 ADAMTS13 mutations.40
Acute episodes can be prevented by regular plasma infusions every 2-3 weeks, although not all patients seem to require prophylaxis because they experience relapses only in situations of increased risk (eg, pregnancy and alcohol binge drinking).38,39 The challenge is to define which patients should be advised to receive regular prophylactic plasma infusions to prevent long-term neurologic and renal sequelae. This is particularly difficult in patients presenting with an adult onset and only infrequent acute episodes, although the latest experimental evidence from an animal model suggests that all hereditary TTP patients may benefit from regular ADAMTS13 replacement because the absence of ADAMTS13 seems to be associated with accelerated atherosclerosis.41 Long-term follow-up data on hereditary TTP cases are therefore urgently needed and constitute a primary goal of the hereditary TTP registry (www.ttpregistry.net; www.clinicaltrials.gov registration number NCT01257269).
The observed plasma half-life of circulating ADAMTS13 (2-3 days) and the empirically determined suitable interval between prophylactic plasma infusions of 2-3 weeks in most patients is discrepant and suggests as yet unknown modes of action or storage reservoirs of infused ADAMTS13. Apart from incidental allergic reactions to infused plasma, supplementation with exogenous ADAMTS13 is generally well tolerated. So far, no alloimmunization with the occurrence of inhibitory ADAMTS13 Abs has been documented in frequently transfused USS patients, although fluctuating titers of anti-ADAMTS13 Abs of IgG isotype measured by ELISA have been observed in several cases.39,42 The significance of these Abs is unknown, especially because recovery and plasma half-life of infused ADAMTS13 were normal in one of these patients.42 Despite the strong pathogenic immune response seen in acquired TTP, plasma-derived ADAMTS13 per se thus seems to have little or no immunogenicity in USS patients. This is in clear contrast to hemophilia A, in which one-quarter to one-third of factor VIII–treated patients develop inhibitory alloantibodies; induction of immune tolerance is an important issue in hemophilia care.
USS is considered an extremely rare disorder. Reliable prevalence and incidence data are missing and frequencies have been based on the number of diagnosed cases in certain areas or on the proportion of hereditary ADAMTS13 deficiency of the whole cohort of severe ADAMTS13-deficient patients in a certain period of time. The large geographic distribution areas of the ADAMTS13 mutations, 4143insA and Arg1060Trp,32,35 the occurrence of homozygous mutations in unrelated families,32,35,38,39 the notion that hereditary TTP is not only a disease of infancy or childhood, and the increasing number of patients recognized with an adult onset of disease suggest that the prevalence of USS, although it remains an orphan disease, may have been underestimated. This is corroborated by the first population screening carried out in Japan, where heterozygous mutations were present in a considerable number of healthy adults stratified according to their plasma ADAMTS13 activity.43 The investigators estimated that the true prevalence of USS could be up to 3-fold higher than the number of patients diagnosed thus far in Japan.
ADAMTS13 and diagnosis and treatment of TTP
The first retrospective studies found a severe ADAMTS13 deficiency in 87%-100% of patients suffering from idiopathic TTP.8,9 Subsequent investigations of less selected cohorts of patients diagnosed clinically with idiopathic TTP reported lower frequencies of severely ADAMTS13-deficient patients (33%-80%). These studies, however, showed that severe ADAMTS13 deficiency was extremely rare in secondary forms of thrombotic microangiopathies, such as those after stem cell transplantation or associated with neoplasia or drugs.44
Although a severe deficiency of ADAMTS13 is not found in all patients diagnosed with idiopathic TTP, ADAMTS13 assays may provide helpful guidelines for treatment. A finding of a severe autoantibody-mediated ADAMTS13 deficiency provides the rationale for adjunctive steroids or treatment escalation with rituximab or splenectomy in plasma refractory or relapsed cases, as well as prognostic information regarding survival and risk of relapse. Nearly half of the survivors initially presenting with an ADAMTS13 activity level < 10% will eventually relapse, with the highest risk in the first 2 years after an acute episode, whereas patients without this deficiency rarely relapse.44 All patients with a prior history of idiopathic TTP due to severe acquired ADAMTS13 deficiency will have severe acquired ADAMTS13 deficiency in a subsequent relapse. Therefore, a severe ADAMTS13 deficiency at any time during remission is associated with an increased risk of relapse,45 although the finding of an ADAMTS13 value < 10% of the normal level is not necessarily a sign of an imminent relapse.44
Citrated plasma (also suitable are serum or heparinized plasma) should be withdrawn for ADAMTS13 testing before the initiation of treatment. Since the identification of the VWF-cleaving protease, several different ADAMTS13 activity assays have been developed. Initially, plasma-derived or recombinant multimeric VWF served as a substrate and required the use of low ionic strength and high concentrations of denaturing substances to unfold globular VWF to make the peptide bond Tyr1605-Met1606 in the VWF A2 domain accessible to cleavage by ADAMTS13. These assays were laborious, had turnaround times of 2-4 days, and were restricted to a few specialized laboratories. Newer, “second-generation” assays use a VWF A2 domain peptide of 73 amino acids identified as the VWF minimal substrate for ADAMTS1346 or a somewhat larger derivative thereof. These assays, including several commercial assays, are easy to perform, robust, and have short turnaround times. The latter is important because getting patient samples to laboratories offering these assays has become the time-limiting factor. Agreement between assays using multimeric VWF substrate and assays using VWF A2 domain peptides as substrate is generally good, but there are some patients in whom a severe ADAMTS13 deficiency by one assay may be associated with an only moderately or mildly reduced ADAMTS13 activity by the other assay.44 It should be kept in mind that even present-day peptide substrate assays do not reflect the short interaction time of ADAMTS13 and multimeric VWF in vivo. Therefore, a normal or mildly reduced ADAMTS13 activity by contemporary assays does not necessarily preclude a causal role of ADAMTS13 in a patient's clinical scenario. Exemplary to this phenomenon is the remarkable course of a patient over 8 years and 6 acute TTP episodes.47 Despite anti-ADAMTS13 Abs demonstrable by ELISA, dot blot, and immunoprecipitation during all 6 episodes, ADAMTS13 activity was normal by 3 different assays in his first episode. During subsequent acute TTP episodes, severe ADAMTS13 deficiency was observed first by a flow-chamber assay, which gets close to the in vivo situation,48 and in later episodes by an initial rate assay using a peptide substrate, and finally by a static end-point assay using multimeric VWF substrate.47 Caution is thus warranted when using in vitro ADAMTS13 activity > 10% as a single criterion for the distinction between idiopathic TTP and other thrombotic microangiopathies.
ADAMTS13 inhibitors are assessed by classical mixing studies of heat-inactivated patient plasma and a normal plasma pool. Results are expressed as Bethesda units per milliliter. In addition, anti-ADAMTS13 Abs can be detected by Western blotting or ELISA using recombinant ADAMTS13 as antigen. In contrast to research assays, many of the commercial ELISAs that are now widely used detect ADAMTS13 Abs in 13%-15% of healthy controls, limiting the utility of these assays to distinguish hereditary from acquired ADAMTS13 deficiency or to identify patients at risk of imminent recurrence after an acute episode of acquired TTP.
Conclusions
The fascination with TTP, a rare but exemplary disease, has remained high over several decades (Figure 3) and recombinant ADAMTS13, which will be available in the near future, will add to this fascination. This will change the life of patients diagnosed with USS and possibly also have an impact on the treatment of patients diagnosed with acquired idiopathic TTP, in whom neutralization of functional inhibitors with recombinant ADAMTS1349 or, even better, with a recombinant ADAMTS13 gain-of-function variant,50 is feasible, at least in vitro. Recombinant ADAMTS13 may also become useful for other diseases in which extensive interaction between VWF and platelets plays an imminent pathophysiologic role, such as myocardial infarction or cerebrovascular stroke, which are not seldom important features in acute TTP episodes.
Acknowledgments
The authors thank Monica Schaller, Irmela Sulzer, Gabriela Mäder, Carlo R. Largiadèr, Sara C. Meyer, Rahel Fröhlich-Zahnd, Jan-Dirk Studt, and Magnus Mansouri Taleghani for work in our laboratory and the members of the Steering Committee of the Hereditary TTP Registry and our long-term collaborators for their continued support and input (in alphabetical order): Ulrich Budde, Hamburg, Germany; Jing-Fei Dong, Seattle, WA; Tobias A. Fuchs, Boston, MA; Yoshihiro Fujimura, Nara, Japan; Miha Furlan, Bern, Switzerland; James N. George, OK; Ingrid Hrachovinova, Prague, Czech Republic; Anne-Sophie von Krogh, Trondheim, Norway; Masanori Matsumoto, Nara, Japan; Toshiyuki Miyata, Osaka, Japan; Petter Quist-Paulsen, Trondheim, Norway; Barbara Plaimauer, Vienna, Austria; Inge Scharrer, Mainz, Germany; Fritz Scheiflinger, Vienna, Austria; Reinhard Schneppenheim, Hamburg, Germany; Deirdra R. Terrell and Sara K. Vesely, OK; Jan Voorberg, Amsterdam, The Netherlands; Denisa D. Wagner, Boston, MA; and X. Long Zheng, Philadelphia, PA. Due to space constraints we were unfortunately not able to cite every relevant paper.
Our research is supported by grants from the Swiss National Science Foundation (32003B-124892), the International Society on Thrombosis & Haemostasis 2007 Presidential Fund, and by Baxter Healthcare for our hereditary TTP registry.
Disclosures
Conflict-of-interest disclosure: J.A.K.H. and B.L. have each received research funding from and consulted for Baxter Healthcare. Off-label drug use: None disclosed.
Correspondence
J. A. Kremer Hovinga, MD, Department of Hematology and Central Hematology Laboratory, Inselspital, Bern University Hospital and the University of Bern, CH-3010 Bern, Switzerland; Phone: 41-31-632-90-22; Fax: 41-31-632-18-82; e-mail: johanna.kremer@insel.ch.