Abstract
Evaluation and management of patients with suspected thrombotic thrombocytopenic purpura (TTP) continue to be a critical challenge for hematologists. The diagnostic criteria are not precise, often causing uncertainty about whether it is appropriate to initiate plasma exchange (PEX), the essential treatment for TTP. Initiation of PEX remains a clinical decision; severe ADAMTS13 (< 10% activity) deficiency alone is neither sufficiently sensitive nor specific for the diagnosis of TTP. However, patients who do have severe acquired ADAMTS13 deficiency define the characteristic clinical features of TTP, the response to treatment, and the long-term outcomes. Patients with severe acquired ADAMTS13 deficiency are predominantly young women and the relative frequency of blacks is increased. Patients may present with only microangiopathic hemolytic anemia and thrombocytopenia, neurologic and renal abnormalities are often not present, fever rarely occurs; the complete “pentad” of these clinical features almost never occurs in current practice. Response to PEX is typically rapid but may not be sustained when PEX is stopped. Use of corticosteroids and rituximab has decreased the number of PEX treatments required to achieve a remission and has resulted in fewer PEX-related major complications. Relapse (in approximately 40% of patients) may be the most apparent risk after recovery, but long-term health outcomes are also very important. Minor cognitive abnormalities are common, the frequency of depression is increased, and the frequency of hypertension is increased. Careful long-term follow-up of TTP patients is essential.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a challenging disorder for hematologists because: (1) the diagnostic criteria are not precise; (2) survival is only 10% without plasma treatment; and (3) although plasma exchange (PEX) treatment allows 80% survival, it is associated with a high risk of major complications.1–5 Recognition of the role of ADAMTS13 has helped our understanding of the pathogenesis of TTP; however, the initial evaluation and management decisions still require clinical judgment. The critical decision is to determine whether the probability of the diagnosis of TTP is sufficient to justify the risks of PEX treatment. ADAMTS13 activity measurements may not help this initial decision. Although the presence of severe ADAMTS13 deficiency (< 10%) is characteristic of TTP, it is neither sufficiently sensitive nor specific to determine the decision to initiate or withhold PEX. However, there may be circumstances in which the presence or absence of severe ADAMTS13 deficiency together with all of the clinical data may be able to contribute to the decision to initiate or withhold PEX. Current diagnostic criteria remain the inclusion criteria for a randomized clinical trial that documented the effectiveness of PEX: the presence of microangiopathic hemolytic anemia and thrombocytopenia without a clinically apparent alternate etiology.2 These limited diagnostic criteria not only make the initial evaluation and management decisions difficult, they also make the names used to describe these syndromes imprecise. All syndromes described by the terms TTP, hemolytic uremic syndrome (HUS), and thrombotic microangiopathy fit within the diagnostic criteria for TTP, but not all of these syndromes are appropriately treated with PEX.
Clinical definitions of the TTP and HUS syndromes
Table 1 presents the traditional definitions for TTP and HUS that reflect common clinical usage for the past 30 years. Acquired TTP occurs predominantly among adults; it is rare among children and the treatment is PEX. Hereditary TTP is much less common than acquired TTP, and for this, plasma infusion is sufficient. HUS is traditionally considered to be a syndrome of children. Almost all children with HUS have preceding diarrhea caused by an enterohemorrhagic infection with Escherichia coli O157:H7; treatment is supportive care and PEX is rarely used.6 Children without a diarrhea prodrome are described as “atypical. ” Among the children with atypical HUS (aHUS), some had a family history of HUS; patients in these families were discovered to have hereditary abnormalities of complement regulation. Therefore, aHUS has recently become the name for syndromes in children or adults caused by complement regulatory abnormalities. PEX may be appropriate for patients with aHUS.7 Adults may have severe microangiopathic hemolytic anemia and thrombocytopenia caused by infection with E coli O157:H7 or by complement regulatory abnormalities, or they can present with acute anuric kidney injury caused by quinine-dependent Abs. These patients' disease may be described as HUS or with the comprehensive term, TTP-HUS; they may be critically ill and therefore PEX is appropriate.
Common clinical nomenclature for syndromes characterized by microangiopathic hemolytic anemia and thrombocytopenia without another apparent etiology

TTP-HUS is a name sometimes used for adults with acute renal failure to emphasize the predominant kidney involvement (eg, patients with quinine-induced TTP-HUS).
The term thrombotic microangiopathy is not included in Table 1; it is a descriptive term for the characteristic pathology of TTP, HUS, and also many other disorders such as syndromes after hematopoietic stem cell transplantation,8 preeclampsia,9 systemic lupus erythematosus (SLE),10 systemic infections,11 systemic malignancies,12 and malignant hypertension.13 PEX is not appropriate when an alternative etiology for the microangiopathic hemolytic anemia and thrombocytopenia, such as systemic infection, systemic malignancy, or malignant hypertension, is discovered.
The Oklahoma TTP-HUS Registry
The data presented here are from the Oklahoma TTP-HUS Registry, an inception cohort of all consecutive patients for whom PEX treatment was requested for a diagnosis of TTP or HUS at all hospitals in our region since 1989, without selection or referral bias.3 A request for PEX treatment was the only inclusion criterion, there were no exclusions; therefore patients who were subsequently discovered to have an alternative explanation for their presenting clinical features remain in the Registry. This single inclusion criterion and the absence of any exclusion criteria make this patient cohort distinct from other reported case series. It may be assumed that in other case series, a decision about whether or not a patient actually has TTP or HUS may be the inclusion criterion. We consider that such a decision is imprecise; therefore we exclude no patients from the Registry and we continue to follow all patients. From 1989 through 2011, 427 patients with their first episode of clinically diagnosed TTP or HUS were enrolled. ADAMTS13 activity has been measured immediately before the initial PEX since November 13, 1995; through 2011, ADAMTS13 activity was measured in 311 (93%) of 333 patients at the time of their initial episode; 70 (23%) patients had severe ADAMTS13 deficiency (< 10% activity); 57 (81%) of these patients survived. The Registry is approved by the institutional review boards of the University of Oklahoma Health Sciences Center and each participating hospital.
Categories of patients who had an initial clinical diagnosis of TTP or HUS and a request for PEX treatment
Table 2 presents the clinical categories of Registry patients. Patients are assigned to one of these clinical categories at the time of their initial episode in a hierarchical manner, beginning at the top of the list in Table 2. If the patient is not included in any of the previous categories, she is described as idiopathic. This is probably similar to patient descriptions in other published case series; it emphasizes the extreme heterogeneity among all patients for whom PEX is requested as well as the heterogeneity of patients who are described as idiopathic. The heterogeneity of patients described as idiopathic is also emphasized by the observation that only 46% had severe ADAMTS13 deficiency. Patients with severe ADAMTS13 deficiency occurred in almost all clinical categories. In patients with a stem cell transplant, sepsis, or a systemic malignancy, the presence of severe ADAMTS13 deficiency did not appear to indicate the presence of TTP; 2 of these 6 patients had autopsies and microvascular thrombi were not observed. In patients who were pregnant or postpartum, who presented with bloody diarrhea, or who had a preceding diagnosis of an autoimmune disorder, the presence of severe ADAMTS13 deficiency did appear to support the diagnosis of TTP. A prodrome of bloody diarrhea may suggest infection with E coli O157:H7, but bloody diarrhea can also be caused by the ischemic colitis resulting from microvascular thrombi.14 The association of TTP and SLE is also apparent; both occur predominantly in young, black women.15 Although the number of patients enrolled has been different from year to year, there has been no consistent change of the number of patients or of the pattern of referrals for PEX treatment, with the exception that fewer patients after hematopoietic stem cell transplant have been referred in recent years. The fraction of patients referred for PEX who have severe ADAMTS13 deficiency has not consistently changed across the past 16 years.
Frequency of severe ADAMTS13 deficiency (activity < 10%) among patients for whom PEX treatment was requested for an initial clinical diagnosis of TTP or HUS: the Oklahoma TTP-HUS Registry experience, 1995-2011
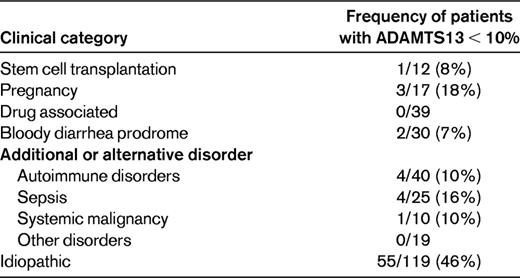
From November 13, 1995 through December 31, 2011, 301 (93%) of 333 consecutive patients enrolled in the Registry had ADAMTS13 activity measured in a sample obtained immediately before beginning the initial PEX by both FRETS and immunoblotting assays.3
Clinical characteristics of patients with severe ADAMTS13 deficiency
Although patients with severe ADAMTS13 deficiency represent only 23% of all Registry patients enrolled for their initial episode of TTP, they are important because their demographics, clinical features, response to treatment, and long-term outcomes define what we often consider to be the characteristics of TTP. Among the 70 patients with severe ADAMTS13 deficiency, the median age was 40 years (range, 9-71 years), 56 (80%) were women, and 24 (34%) were black. Their presenting clinical features are described in Table 3. All patients had thrombocytopenia and microangiopathic hemolytic anemia, the diagnostic criteria for TTP. Approximately one-third had severe neurologic abnormalities (defined as coma, stroke, seizure, or transient focal abnormalities); one-third had minor neurologic abnormalities (typically confusion); one-third had no neurologic abnormalities. Only 6 (9%) patients had acute renal failure and 29 (41%) had a serum creatinine ≥ 1.5 mg/dL at some time during their course; in half of the patients the serum creatinine was always < 1.5 mg/dL. Only 15 (21%) of patients had fever. Most importantly, the 3 patients who had the complete “pentad” of clinical features included one patient who was subsequently discovered to have Group A streptococcal sepsis, one patient with fulminant hepatitis A (who did not have evidence for TTP at autopsy), and one patient with preceding SLE. Therefore, it is very clear that the pentad of presenting clinical features that was introduced before the era of PEX treatment, when the mortality of TTP was 90%,1 is obsolete.
Presenting clinical features of 70 consecutive patients with severe ADAMTS13 deficiency (activity < 10%)
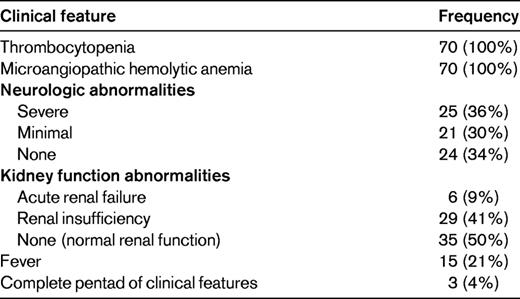
From November 13, 1995 through December 31, 2011, 70 (23%) of all 301 patients in whom ADAMTS13 activity was measured had activity < 10% by either the FRETS or immunoblotting assay or both. The data describe the presenting clinical features of these 70 patients. Severe neurologic abnormalities were defined as transient focal abnormalities, seizures, stroke, or coma; minimal abnormalities included headache and minor mental status changes such as transient confusion. Acute renal failure was defined as an increased serum creatinine of ≥ 0.5 mg/dL/d for 2 consecutive days or a serum creatinine ≥ 4.0 mg/dL with dialysis. Renal insufficiency was defined as any serum creatinine value ≥ 1.5 mg/dL.27
Management of patients with severe ADAMTS13 deficiency
PEX has been the essential and urgent treatment for patients with a clinical diagnosis of TTP since the publication of the randomized clinical trial by the Canadian Apheresis Group in 1991.2 Treatment with PEX increased the survival of patients from 10%1 to 78%2 ; survival of patients with severe ADAMTS13 deficiency remained 78% through 2009.3 The risks of PEX are substantial. From 1996-2011, we have collected data on 302 consecutive patients treated with PEX for their first episode of clinically diagnosed TTP or HUS5 using definitions described in our initial publication.16 Ninety-four major complications have occurred in 72 (24%) patients. This is a higher frequency of major complications than is often suggested, probably because our data are collected from the time the central venous catheter is inserted (before PEX is begun) and continues after PEX is stopped (often revealing sepsis and/or venous thrombosis related to the central venous catheter). We have analyzed data on PEX complications every 3 years. When data for the fifth 3-year cohort were analyzed in 2011, we made the unexpected observation that the frequency of PEX complications had decreased significantly, primarily because of the significantly decreased major complications related to the central venous catheters.5 We then analyzed patients with severe ADAMTS13 deficiency (activity < 10%) separately from patients with ADAMTS13 activity ≥ 10%; the trend for decreasing frequency of PEX-related major complications was significant for patients with severe ADAMTS13 deficiency but not for patients with ADAMTS13 activity ≥ 10%.5 The trend for decreasing PEX-related major complications in patients with severe ADAMTS13 deficiency was consistent with the decreasing days of PEX required to achieve a remission and the increasing frequency of adjunctive treatments with corticosteroids and rituximab (Table 4).5 The increasing use of corticosteroids and rituximab is consistent with our published recommendations: in 2000, corticosteroids were not used routinely and rituximab was not available.17 In 2010, corticosteroids were used in all patients in whom severe ADAMTS13 deficiency was suspected; rituximab was used in patients with a poor initial response to treatment with PEX and corticosteroids or in whom an exacerbation of TTP occurred when PEX was stopped.4 These indications for rituximab use are similar to a report from the French Thrombotic Microangiopathies Network.18
Changes of the frequency of major complications of PEX, days of PEX treatment, and frequency of treatment with corticosteroids and rituximab in patients with their initial episode of TTP associated with severe ADAMTS13 deficiency, 1996-2011
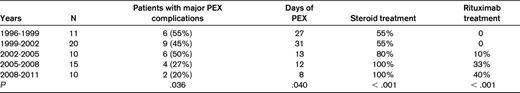
PEX complications have been documented as described previously16 in all Registry patients from June 25, 1996 through June 24, 2011. Results have been analyzed every 3 years. The data describe the results of the 5 consecutive 3-year periods for 66 patients with ADAMTS13 activity < 10%. Of the 70 patients described in Table 3, 1 patient died before a central venous catheter was placed for PEX, 2 patients presented before June 25, 1996, and 1 patient presented after June 24, 2011. A 1-sided Cochran-Armitage Trend test was performed to determine whether the proportion of patients with major complications, days of PEX, or treatment with steroids or rituximab changed across the 5 time periods. Adapted with permission from Som et al.5
Since 2003, 10 patients with severe ADAMTS13 deficiency have been treated with rituximab during their initial episode; 1 patient died and 1 (11%) of the 9 survivors relapsed at 2.6 years (8 days after delivery of a healthy baby and a pregnancy complicated by mild preeclampsia); the remaining 8 patients have been followed for a median of 4.8 years (range, 1.5-7.6 years). Comparison of patients treated or not with rituximab during their initial episode demonstrated that the time to first relapse was not significant. We have treated 11 additional patients with rituximab for a relapsed episode. Using each of these 11 patients as their own control, the frequency of relapses per patient-years at risk before rituximab (0.277/y) was greater than after rituximab treatment (0.045/y), but the difference was not significant. Our experience suggests that rituximab may delay but not prevent the occurrence of relapses, although with more patients and longer follow-up, the difference between the frequency of relapse for patients treated with rituximab may become significantly less than for patients not treated with rituximab.
A recent report on rituximab as initial treatment for patients with TTP suggested that rituximab may be considered as routine initial treatment.19 However, this study had several issues with patient selection and analysis that limit the strength of this suggestion. For example, the selection of the control patients was incompletely described and may not have been blinded, matching of treated to control patients was not ideal, the duration of follow-up for each patient group was not defined, censoring of patients in the analysis of relapse-free survival was not reported, and not all patients had severe ADAMTS13 deficiency.19 Because many patients with severe ADAMTS13 deficiency achieve a remission promptly with only PEX and corticosteroids and most never relapse, the appropriate use of rituximab cannot be determined from current data.
Platelet transfusions
Although the danger of platelet transfusions has been a firm conviction of hematologists for decades, and although the biologic rational for this conviction is apparent, platelet transfusions may not be dangerous. The original description of death after a platelet transfusion was dramatic, occurring in a patient before the era of PEX treatment.20 The danger of platelet transfusions has been supported by subsequent anecdotes, but an adverse effect of platelet transfusions has not been apparent in the many case series of patients with TTP.21 In our experience, 33 (61%) of 54 patients with severe ADAMTS13 deficiency received one or more platelet transfusions, most before the diagnosis of TTP was considered or established. The mortality and the occurrence of severe neurologic events was not different between patients who had or had not received a platelet transfusion.21 Therefore, the appropriate use of platelet transfusions for a patient with severe thrombocytopenia and overt bleeding or for a patient who needs a major invasive procedure is also appropriate for patients with TTP. These situations rarely occur in patients with TTP. Our practice is to advise that a platelet transfusion is unnecessary for central venous catheter insertion.
Long-term outcomes of patients with severe ADAMTS13 deficiency
Relapse is the most apparent risk after recovery and is almost totally restricted to patients with severe ADAMTS13 deficiency, among whom the estimated risk at 7.5 years is 43%.3 Table 5 lists the long-term outcomes in the 57 of 70 patients with severe ADAMTS13 deficiency who survived their initial episode of TTP. The morbidity and mortality from relapsed episodes is less than with initial episodes because there is no delay in establishing the diagnosis and beginning treatment. To ensure rapid diagnosis and treatment, we insist that our patients have a complete blood count when they have any symptoms of illness and see their hematologist immediately if their platelet count is low. Pregnancy has often been cited as a risk for recurrence of TTP because most case reports of pregnancies after recovery from TTP describe the occurrence of relapse.22 However, this is inevitably biased by the reporting of complicated patients, with less interest in reporting patients with uncomplicated pregnancies. Among our patients who have recovered from TTP associated with severe ADAMTS13 deficiency, 8 women have had 15 subsequent pregnancies; 2 (13%) pregnancies have been associated with a recurrent episode of TTP. One patient had 2 uncomplicated pregnancies after her recovery; her second episode of TTP occurred 38 days after her second delivery. One patient had had 2 uncomplicated pregnancies before her initial episode of TTP; 2.6 years after she recovered, she had a cesarean section because of mild preeclampsia and TTP recurred 8 days after delivery. We do not discourage women from becoming pregnant after recovery from TTP, but we strongly encourage careful prenatal evaluations with frequent platelet counts and frequent evaluations after delivery.
Long-term outcomes in the 57 of 70 patients with severe ADAMTS13 deficiency who survived their initial episode of TTP
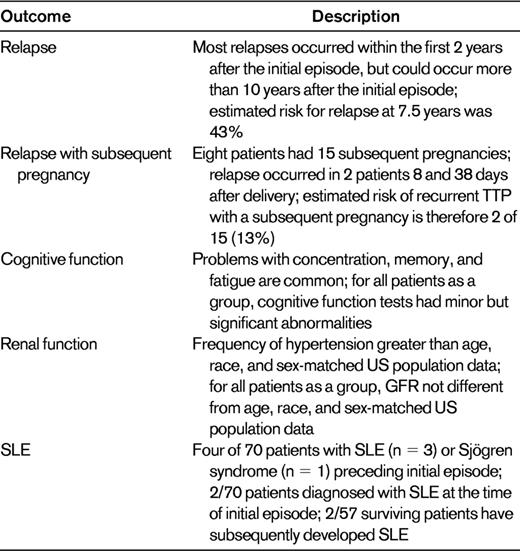
Data and interpretations are derived from all 57 (81%) survivors of the 70 patients who presented with ADAMTS13 activity < 10%, 1995-2011.
GFR indicates glomerular filtration rate.
Perhaps the most common concern of patients after recovery is minor cognitive abnormalities that cause problems with concentration, memory, and fatigue.23 Some patients feel that they have recovered completely and have no cognitive problems, but most patients (or their spouses) state that their recovery has not been complete.24,25 Although these abnormalities are subtle—all patients have returned to their previous professions and activities—the presence of these minor cognitive abnormalities can have an important impact on quality of life.26 In addition, our preliminary data suggest that the frequency of depression is significantly increased after recovery from TTP.
Other subtle abnormalities can cause significant long-term health problems for patients after recovery from TTP. Renal function is not different from age-, race-, and sex-matched subjects in the National Health and Nutrition Examination Survey (NHANES) databases. This may be related to our observations that the frequency of hypertension requiring treatment is also significantly greater than age-, race-, and sex-matched subjects in the NHANES databases. These abnormalities may be related to residual organ dysfunction after TTP or to the characteristics of patients who have TTP associated with severe ADAMTS13 deficiency. The frequency of obesity is significantly increased among these patients,27 increasing their risk factors for developing diabetes and hypertension. The increased frequency of SLE among patients with TTP associated with severe ADAMTS13 deficiency probably reflects the similar demographics of patients with TTP and SLE (both occur predominantly in young black women) and also the increased risk for autoimmune disorders in patients with a previous autoimmune disorder.
Conclusions
The diagnosis of TTP may be difficult. Among patients with severe acquired ADAMTS13 deficiency, the previously promoted pentad of presenting clinical features rarely occurs; many patients have only thrombocytopenia and microangiopathic hemolytic anemia. Management of patients with corticosteroids in addition to PEX, and rituximab in selected patients, has significantly decreased the time required to achieve remission. After recovery from TTP, careful follow-up is essential, not only to ensure prompt diagnosis and treatment of a relapsed episode of TTP, but also to ensure proper management of the patient's physical and mental health. Perhaps the most important area for future research is to more accurately define the long-term outcomes of patients after their recovery from acute episodes of TTP.
Disclosures
Conflict-of-interest disclosure: J.N.G. serves as a consultant for Baxter for the development of rADAMTS13 and is on the advisory board of Alexion for eculizumab. Z.L.A.-N. declares no competing financial interests. Off-label drug use: rituximab for TTP.
Correspondence
James N. George, MD, The University of Oklahoma Health Sciences Center, Hematology, Rm CHB-237, PO Box 26901, Oklahoma City, OK 73126-0901; Phone: 405-271-4222; Fax: 405-271-6444; e-mail: james-george@ouhsc.edu.
