Abstract
Thrombosis is a leading cause of morbidity and mortality in patients with Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs), particularly polycythemia vera and essential thrombocythemia. Mechanisms involved in the pathogenesis of the acquired thrombophilic state associated with these diseases include abnormalities of MPN clone–derived blood cells, which display prothrombotic features, and abnormalities of normal vascular cells, which become procoagulant in response to inflammatory stimuli. Ultimately, the release into the blood of elevated levels of procoagulant microparticles by platelets and vascular cells and the increase in the global thrombin generation due to an acquired activated protein C resistance result in a highly prothrombotic scenario in patients with polycythemia vera and essential thrombocythemia. The acquired point mutation in the pseudokinase domain of JAK2 (JAK2V617F) in these disorders is variably associated with thrombosis and, more consistently, with elevations in WBC counts and alterations in biomarkers of blood-clotting abnormalities. The predictive value of these biomarkers for thrombosis remains to be established to identify subsets of patients at elevated risk who may benefit from prophylaxis with antithrombotic drugs.
Introduction
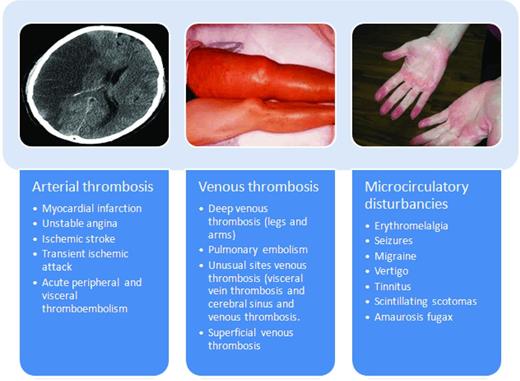
Myeloproliferative neoplasms (MPNs) are clonal disorders of hematopoietic stem cells characterized by proliferation of one or more myeloid cell lines (granulocytic, erythroid, megakaryocytic, and mast cells). According to the World Health Organization (WHO) classification, these include classic MPN Philadelphia chromosome (Ph)–negative diseases which include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). The discovery of recurrent molecular abnormalities such as JAK2V617F and MPL W515L/K has reinforced the original idea of Dameshek in 1951 that these diseases share a common pathogenetic mechanism and presumably belong to a single disorder. Clonality studies have established that PV, ET, and MF are diseases that originate from a common hematopoietic stem cell that is hypersensitive to the action of growth factors. The result is an autonomous proliferation of hematopoietic colonies, which leads to overproduction of red blood cells (RBCs), platelets, and leukocytes. Among these disorders, ET has the most favorable outcome. Patients with ET have a lifespan that nearly rivals that of a healthy population matched by age and sex. However, the life expectancy of patients with PV and ET is strongly affected by disease-related hemostatic complications, including thrombosis and, to a lesser extent, hemorrhages. Reported incidence of thrombosis ranges from 12%-39% in PV and from 11%-25% in ET.1 Clinical manifestations of thrombosis vary from mild microcirculatory disturbances to more serious complications such as arterial and venous thrombosis (Figure 1).
The spectrum of thrombotic manifestation in ET and PV. Thrombophilia, which severely affects the morbidity and mortality of PV and ET, is variably characterized by microcirculatory disturbances and arterial and venous thromboses that often precede disease recognition. Thrombotic occlusions of large arteries most commonly involve cerebral or coronary vessels. Ischemic stroke constitutes 30%-40% of all thrombotic events in PV patients. Acute coronary syndromes are more rare, particularly during followup of treated patients. Acute vascular occlusions in other areas are not uncommon both in PV and ET patients. Venous thrombosis usually manifests with an increased incidence of deep venous thromboses of the lower limbs, which may cause pulmonary embolism. Superficial phlebitis of the legs is also common and venous thromboses at unusual sites are not as rare as in the general population. Thromboses of cerebral sinuses and of splanchnic (portal and hepatic) veins have been repeatedly reported in relatively young female patients. Microcirculatory disturbances are the most peculiar thrombotic manifestations in PV and ET patients and are responsible for a wide range of clinical symptoms arising from the formation of platelet thrombi in the end-arterial circulation of the peripheral, cerebral, coronary, skin, and abdominal vessels.
The spectrum of thrombotic manifestation in ET and PV. Thrombophilia, which severely affects the morbidity and mortality of PV and ET, is variably characterized by microcirculatory disturbances and arterial and venous thromboses that often precede disease recognition. Thrombotic occlusions of large arteries most commonly involve cerebral or coronary vessels. Ischemic stroke constitutes 30%-40% of all thrombotic events in PV patients. Acute coronary syndromes are more rare, particularly during followup of treated patients. Acute vascular occlusions in other areas are not uncommon both in PV and ET patients. Venous thrombosis usually manifests with an increased incidence of deep venous thromboses of the lower limbs, which may cause pulmonary embolism. Superficial phlebitis of the legs is also common and venous thromboses at unusual sites are not as rare as in the general population. Thromboses of cerebral sinuses and of splanchnic (portal and hepatic) veins have been repeatedly reported in relatively young female patients. Microcirculatory disturbances are the most peculiar thrombotic manifestations in PV and ET patients and are responsible for a wide range of clinical symptoms arising from the formation of platelet thrombi in the end-arterial circulation of the peripheral, cerebral, coronary, skin, and abdominal vessels.
Arterial thrombosis, which accounts for 60%-70% of events related to MPNs, includes ischemic stroke, acute myocardial infarction, and peripheral arterial occlusion. The high incidence of heart valvular lesions in MPNs raises the possibility that a significant proportion of these may be related to emboli of cardiac origin. Events involving the venous system are represented by deep venous thrombosis of the lower extremities, pulmonary embolism, intraabdominal (hepatic, portal, and mesenteric) and cerebral vein thrombosis. In PV, venous thromboses are relatively common and constitute approximately one-third of total events. The prevalence of splanchnic and cerebral vein thrombosis is unusually high among patients with MPN, and these events are often the presenting feature of the disease before diagnosis.2 MPNs constitute the most common cause of splanchnic venous thromboses, accounting for approximately 50% of Budd Chiari syndrome (BCS) cases and 25% of portal vein thromboses (PVT). MPNs associated with BCS and PVT have unique features compared with “classic” MPNs, including the onset at a younger age, female predominance in PV, and the presence of normal blood counts, which sometimes renders the diagnosis of MPN quite challenging.3
Typical of, but not exclusive to, ET is the involvement of the microcirculatory system, which manifests as erythromelalgia, transient ischemic attacks, visual or hearing transitory defects, recurrent headache, and peripheral paresthesia. Focal symptoms such as dysarthria, transient monocular blindness, or transient mono- or hemiparesis are less common. Visual dysfunction may also manifest as transient diplopia and sudden reversible attacks of blurred vision.
ET and PV management remains highly dependent on the patient's thrombotic risk.4 Because the use of myelosuppressive drugs (eg, hydroxyurea) can reduce the rate of thromboses and hemorrhages in these patients, but can also accelerate the rate of leukemic transformation, a risk-oriented management strategy is highly recommended. Older age (> 60 years) and previous thrombosis are well established cardiovascular risk factors for thrombosis in these patients. The absence of both of these 2 risk factors identifies low-risk patients. There is great attention being given to moving beyond these recognized risk factors, particularly in the young or asymptomatic low- and intermediate-risk patients not without risk of thrombosis. Recently, the impact of new risk factors, such as leukocytosis and JAK2V617F mutational status and/or mutational burden, has begun to be under active investigation.
Even in the absence of thrombotic manifestation, ET and PV patients present with a hypercoagulable state, which is a laboratory finding of increased levels of plasma biomarkers of hemostatic system activation.5–6 Therefore, an acquired thrombophilic state develops in these patients, who are prone to vascular complications, but the mechanisms ultimately responsible for blood activation coagulation and the increased thrombotic tendency in ET and PV have not been fully elucidated.
Pathogenesis of thrombosis
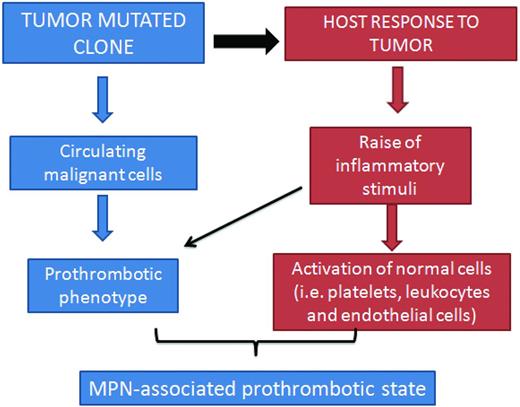
The pathogenesis of thrombosis in ET and PV is multifaceted. However, 2 main mechanisms recapitulate the origin of the hypercoagulable state in these disorders. One relies on the abnormalities of the blood cells (ie, platelets, erythrocytes, and leukocytes) arising from the clonal hematopoietic progenitor cells, which express a prothrombotic phenotype, and the other involves the inflammatory response of host vascular cells to the insult of cytokines and other mediators released by malignant cells (Figure 2).
Pathogenesis of thrombophilia in MPN. The pathogenesis of the acquired thrombophilic state in ET and PV is multifaceted. However, 2 main mechanisms recapitulate the origin of hypercoagulation in these disorders. One relies on the abnormalities of blood cells (ie, platelets, erythrocytes, and leukocytes) arising from the clonal proliferation of hematopoietic progenitor cells, which acquire a prothrombotic phenotype. The other generates from the host inflammatory response to the insult of cytokines and other mediators by the malignant cells. The latter mechanism also contributes to thrombosis in nonmalignant conditions.
Pathogenesis of thrombophilia in MPN. The pathogenesis of the acquired thrombophilic state in ET and PV is multifaceted. However, 2 main mechanisms recapitulate the origin of hypercoagulation in these disorders. One relies on the abnormalities of blood cells (ie, platelets, erythrocytes, and leukocytes) arising from the clonal proliferation of hematopoietic progenitor cells, which acquire a prothrombotic phenotype. The other generates from the host inflammatory response to the insult of cytokines and other mediators by the malignant cells. The latter mechanism also contributes to thrombosis in nonmalignant conditions.
The alterations of circulating MPN clone–derived blood cells entail not only quantitative changes in the number of circulating blood cells, with consequent hyperviscosity, but also qualitative changes that lead to the expression of procoagulant characteristics. The prothrombotic factors expressed by transformed vascular cells (ie, platelets, RBCs, and leukocytes) include the production of procoagulant and proteolytic molecules, secretion of inflammatory cytokines, and expression of cell adhesion molecules. In addition, blood hyperviscosity and increased levels of leukocyte-derived proteases (ie, elastase, cathepsin-G, and myeloperoxidase) may contribute to the thrombophilic state by affecting the integrity of endothelial cell monolayer. Abnormalities of the endothelium do occur in MPN Ph− diseases. Activated/injured endothelial cells express higher levels of adhesion molecules on their surface, which favors platelet and leukocyte arrest, allowing the localized secretion of thrombogenic and angiogenic peptides by inflammatory cells. In this scenario, the increased production of procoagulant microparticles from activated platelets and vascular cells and the increased thrombin generation due to an acquired resistance to activated protein C (APC) represent very important mechanisms that trigger systemic hypercoagulation and ultimately thrombosis in ET and PV patients.
Abnormalities of MPN vascular cells
Platelets.
The role of thrombocytosis in the pathogenesis of thrombotic events is controversial. Although clinical improvement of microcirculatory disturbances and/or improved platelet function after control of thrombocytosis have been reported, no clear correlation of thrombocytosis with risk of major cardiovascular events has been demonstrated.7–8 For example, in both the Polycythemia Vera Study Group (PVSG)9 and the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP)10 prospective trials, platelet count did not predict for thrombosis. The results of the only randomized clinical trial to date, the Primary Thrombocythaemia 1 (PT1) study, which randomized high-risk ET patients to hydroxyurea (a global myelosuppressive agent) or anagrelide (a platelet-only reducing agent), showed that, despite a similar control of platelet count by either drug, the composite primary end point (ie, arterial or venous thrombosis, serious hemorrhage, or death from vascular causes) occurred more frequently in recipients of anagrelide plus aspirin than in those receiving hydroxyurea plus aspirin.11 The global effects of hydroxyurea on blood cell populations other than platelets (eg, leukocytes) might underlie the correlation between control of platelet count and reduction of thrombosis rate observed in the first prospective study in high-risk ET patients by Cortelazzo et al.12 In contrast, in MF, a condition characterized by less frequent cardiovascular events than in PV or ET, a correlation between thrombocytosis and thrombosis was found.13
Conversely, extreme thrombocytosis (ie, platelets > 1500 × 109/L) can favor hemorrhagic rather than thrombotic manifestations in ET patients.12 This paradox has been attributed to the possible occurrence of an acquired VWD due to an increased clearance by platelets of the large circulating VWF multimers.14 Platelet count reduction proved successful in normalizing the plasma VWF multimer pattern and in reducing bleeding tendency.7,8,15
Despite the inconclusive data on the role of thrombocytosis, several other lines of evidence support a contribution of platelets to the pathogenesis of thrombosis in ET and PV. For example, the prompt relief of symptoms with aspirin, together with the normalization of platelet activation tests, suggest a role of platelets in mediating microvessel occlusions (ie, erythromelalgia) in ET patients.16 In contrast to the inefficacy of the oral anticoagulant warfarin, control of platelet function with low-dose aspirin and normalization of platelet counts prevents the recurrence of microvascular circulation disturbances in the end-arterial microvasculature of the cerebral, coronary, and peripheral circulation. Furthermore, low-dose aspirin significantly reduces the risk of cardiovascular events in PV, as demonstrated by the ECLAP randomized clinical trial. Patients randomized to receive aspirin had a 60% reduction of combined end point of nonfatal acute myocardial infarction, nonfatal stroke, or death from cardiovascular causes.10
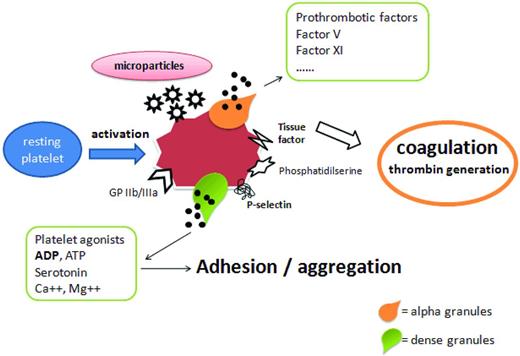
The characteristics of thrombohemorrhagic diathesis in ET and PV prompted the design of many in vitro studies to demonstrate and characterize possible platelet abnormalities. In the past, numerous platelet defects have been identified in ET and PV patients. The majority of these observations were related to a decreased functionality and included abnormal platelet aggregation, reduced levels of membrane adhesion molecules (ie, glycoprotein Ib [GPIb], GPIIb-IIIa, GPIV, and GPVI), acquired storage pool disease, and defective platelet metabolism (ie, abnormal arachidonic acid metabolism).17,18 In contrast, more recent studies show that platelets from these patients circulate in an activated status, as assessed by the detection of increased expression on their surface of P-selectin and tissue factor (TF).19,20 It may be that the permanent activation status of the MPN platelets may exhaust their functions, leading to a reduced response to the stimuli in vitro. We found that although the circulating ET platelets express high surface P-selectin, their response to ADP and epinephrine stimulation is inferior to that of healthy control platelets. Similar dysfunctions in ET and PV platelets were also reported by others.21 Furthermore, some platelet aggregation abnormalities in ET may be caused by laboratory artifacts due to the extensive dilution of platelet-rich plasma needed in the aggregation assay.22 The evidence of enhanced in vivo platelet activation in ET and PV patients is further demonstrated by the finding of increased release of platelet activation products both in plasma (ie, beta-thromboglobulin and platelet factor 4) and urine (ie, the thromboxane A2 [TxA2] metabolites 11-dehydro-TxB2 and 2,3-dinor-TxB2).21,23 Once activated, platelets expose on their surface the anionic phosphatidylserine, usually kept on the inner leaflet (ie, the cytosolic side) of cell membrane, providing a catalytic surface for the generation of thrombin, which further amplifies platelet activation (Figure 3).
Role of platelet abnormalities in MPN-associated thrombophilia. Many studies have investigated the contribution of platelets in the onset of the thrombophilic state in MPNs, and it is now clear that the increased platelet count is not a major element in the risk of thrombosis. Rather, platelet qualitative abnormalities have been implicated in the pathogenesis of hypercoagulability in ET and PV patients. Increased expression of P-selectin, thrombospondin, and the activated fibrinogen receptor GPIIb/IIIa by platelets has been found to be correlated with thrombosis. The formation of platelet-leukocyte aggregates, platelet activation, and microparticle shedding are also implicated in the pathogenesis of thrombosis in these patients. Microparticles expose the anionic phosphatidylserine, providing a catalytic surface for the generation of thrombin, which further amplifies platelet activation.
Role of platelet abnormalities in MPN-associated thrombophilia. Many studies have investigated the contribution of platelets in the onset of the thrombophilic state in MPNs, and it is now clear that the increased platelet count is not a major element in the risk of thrombosis. Rather, platelet qualitative abnormalities have been implicated in the pathogenesis of hypercoagulability in ET and PV patients. Increased expression of P-selectin, thrombospondin, and the activated fibrinogen receptor GPIIb/IIIa by platelets has been found to be correlated with thrombosis. The formation of platelet-leukocyte aggregates, platelet activation, and microparticle shedding are also implicated in the pathogenesis of thrombosis in these patients. Microparticles expose the anionic phosphatidylserine, providing a catalytic surface for the generation of thrombin, which further amplifies platelet activation.
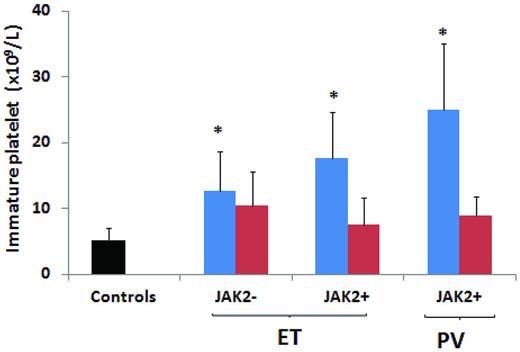
Recently, our group demonstrated in patients with ET and PV, and particularly in carriers of the JAK2V617F mutation, an increased thrombin generation capacity of platelets, which was associated with the occurrence of platelet activation. Cytoreductive therapy with hydroxyurea significantly affected this prothrombotic phenotype.24 We also showed that immature platelets, which are more reactive than their mature counterparts, are also increased in patients with ET or PV (Figure 4) and are significantly down-regulated by hydroxyurea.25 No studies have been performed to compare, in the same patient, platelets with and without JAK2V617F mutation. Conversely, there are data comparing platelets from JAK2V617F+ with those from JAK2V617F− patients and platelets from subjects with different allele mutation burden. In a study of MPN patients by our group, a direct association between JAK2V617F allele burden and increased platelet-associated thrombin generation potential was observed.24
Immature platelets are increased in JAK2V617F+ ET and PV patients. Figure shows data on immature platelet fraction (IPF) values according to disease type, JAK2V617F mutation, and hydroxyurea (HU) treatment. Both ET and PV patients positive for the JAK2V617F mutation and not receiving HU showed significantly higher IPF count compared with control subjects (P < .05). In addition, JAK2V617F+ ET and PV patients not treated with HU showed significantly higher IPF count compared with JAK2V617F− and HU-treated JAK2V617F+ and JAK2V617F− patients not treated with HU. *P < .05 compared with controls. Modified with permission from Panova-Noeva et al.24
Immature platelets are increased in JAK2V617F+ ET and PV patients. Figure shows data on immature platelet fraction (IPF) values according to disease type, JAK2V617F mutation, and hydroxyurea (HU) treatment. Both ET and PV patients positive for the JAK2V617F mutation and not receiving HU showed significantly higher IPF count compared with control subjects (P < .05). In addition, JAK2V617F+ ET and PV patients not treated with HU showed significantly higher IPF count compared with JAK2V617F− and HU-treated JAK2V617F+ and JAK2V617F− patients not treated with HU. *P < .05 compared with controls. Modified with permission from Panova-Noeva et al.24
It is relevant to discuss whether thrombopoietin (TPO) and its receptor (c-MPL) may play a role on platelet function relative to the presence of the JAK2V617F mutation. A decreased expression of c-MPL on platelets and megakaryocytes surface is an established characteristic of PV and MF, but not of ET. Very recently, it was shown that c-MPL expression is down-regulated in JAK2V617F+ platelets in MF due to an increased c-MPL degradation, leading to a megakaryocytic cell–enhanced cell survival and proliferation. However, very little information is available on the possible relationship between TPO/cMPL and the hemostatic platelet functions. There is evidence that TPO induces a potentiation of platelet aggregation and dense-granule secretion after standard stimuli (eg, collagen, ADP, and epinephrine) in patients with MPN.26 However, relationships between c-MPL levels, TPO levels, JAK2V617F mutation, and platelet hemostatic function have not been explored as yet. It may be possible that the constitutive activation of c-MPL in platelets positive for JAK2V617F may render these cells more reactive to stimuli and determine an increased expression of P-selectin.
RBCs.
The prothrombotic effect of an elevated hematocrit has been clearly demonstrated in PV by the observation that a progressively higher hematocrit corresponds to an increase in the thrombotic risk.27 Hematocrit levels around the upper limit of normal values may be an important factor in the causation of occlusive vascular diseases, particularly in the cerebral circulation, as a consequence of an increased blood viscosity. Indeed, at high hematocrit values (47%-53%), the cerebral blood flow is significantly slower than at hematocrit values in the lower range (36%-46%). In untreated PV subjects, most thrombotic accidents occur in the cerebral circulation, which is particularly sensitive to blood hyperviscosity. At high shear rates, the raise of RBC mass displaces platelets toward the vessel wall, thus facilitating shear-induced platelet activation and enhancing platelet-to-platelet interactions. In addition, at low shear rates, as in the venous bed, hyperviscosity can increase the thrombotic risk by causing a major disturbance to the blood flow. A proper management of blood hyperviscosity is essential but does not abolish the in vivo platelet activation and the increased thrombotic risk in PV subjects.23 In addition to an increased RBC count, biochemical changes in cell membrane and intracellular content of RBCs are also found in ET and PV.28 These changes may independently impair blood flow through the formation of RBC aggregates that have the potential to block blood flow in small vessels directly and facilitate platelet-leukocyte interaction. This likely contributes to ischemia and infarct, especially in the cerebral blood flow.
Leukocytes.
Many retrospective studies have identified leukocytosis as a potential risk factor for arterial and venous thrombosis in patients with ET and PV.29–32 Leukocytosis was also a risk factor for recurrent arterial thrombosis in young (ie, < 60 years) ET and PV patients (hazard ratio for arterial recurrence = 3.35, 95% confidence interval [CI], 1.22-9.19).33 In contrast, leukocytosis at diagnosis (defined by a cutoff level of either 15 or 9.4 × 109/L) did not appear to influence the risk of thrombosis in a retrospective cohort of low-risk ET or PV patients.34 Prospective clinical studies with stratification of patients according to their baseline leukocyte counts are needed to definitely classify leukocytosis as a prognostic risk factor. Leukocytes can contribute to the pathogenesis of thrombosis in ET and PV through recently discovered mechanisms of activation and interaction with platelets, endothelial cells, and the coagulation system. In addition, leukocytes contribute to inflammatory processes in atherosclerotic plaques and in this way increase the probability of vascular events. Because neutrophils represent the most abundant proportion of the circulating leukocytes, a role for neutrophils in thrombosis of MPN has been hypothesized. Neutrophils have a central role in the inflammatory response and also in linking the inflammatory response to the activation of blood coagulation.35 Once activated, neutrophils produce reactive oxygen species, release proteolytic enzymes from their cytoplasmic azurophilic granules (ie, elastase and cathepsin G), and express higher and functional levels of the β2 integrin Mac 1 (or CD11b) on their cell surface. All of these molecules can affect the hemostatic system and induce a prothrombotic condition.36 The fact that activated neutrophils can induce a hypercoagulable state in vivo has been well demonstrated by a study of a group of healthy donors administered G-CSF for the mobilization and collection of peripheral blood progenitor cells.37 G-CSF caused activation of neutrophils in these subjects, which was associated with a parallel increment in plasma levels of biomarkers of activation of blood coagulation and endothelium. These effects were transient, persisting only as long as the growth factor was administered (ie, 5-6 days), and normalized after G-CSF withdrawal.
In ET and PV patients, neutrophil activation has been demonstrated by detection of specific phenotypical changes (by increments in membrane-associated CD11b) and of increased plasma concentration of neutrophil granule–derived proteases (ie, elastase and myeloperoxidase).5,19 These abnormalities are directly correlated with increased plasma levels of biomarkers of both blood clotting and endothelium activation, supporting a possible involvement of neutrophils in the pathogenesis of the hypercoagulable state in these disorders. Several studies described increased levels of circulating platelet-neutrophil aggregates in ET and PV patients and attributed this phenomenon to both platelet and neutrophil activation.38 Interestingly, in patients receiving aspirin, the increments in CD11b expression and neutrophil/platelet aggregates induced by in vitro stimulation were significantly lower compared with nonaspirin-treated subjects, suggesting that aspirin treatment may inhibit the interaction between neutrophils and platelets.19 Because the G-CSF receptor is linked to the JAK2 pathway, it is possible that the constitutive activation of signaling through this receptor in the presence of JAK2V617F mutation can be in part responsible for the activated phenotype of neutrophils in patients with ET and PV. However, our data on neutrophil activation analyzed according to the JAK2V617F mutation39 show that the levels of only some of the activation markers are more altered in the JAK2V617F+ than in the JAK2V617F− ones (ie, CD14 and leukocyte alkaline phosphatase), whereas others, (ie, CD11b and plasma elastase) are equally elevated in both groups. It is likely that mechanisms independent of the JAK2V617F mutation are present in non-JAK2V617F mutation carriers.
Abnormalities of host vascular cells
Endothelium.
Physiologically, the endothelium facilitates blood flow by providing an antithrombotic surface that inhibits platelet adhesion and coagulation activation. Several factors may perturb the resting state of endothelium in patients with MPN and turn it into a proadhesive and procoagulant surface. Reactive oxygen species and intracellular proteases released by activated neutrophils can induce detachment or lysis of endothelial cells, affecting functions involved in thromboregulation. It has also been demonstrated that damage of endothelium determines the release in circulation of specific markers including thrombomodulin, selectins, and VWF. This is of particular relevance in the pathogenesis of thrombosis in MPN because once platelets bind to VWF, they become activated and able to aggregate and strengthen the clot.40 Selectins are a family of adhesion molecules expressed by endothelial cells (P-selectin and E-selectin), platelets (P-selectin), and leukocytes (L-selectin).41 Because they are released into the circulation, their presence has been used as an index of endothelial, platelet, and leukocyte activation. In patients with ET and PV, we measured increased plasma levels of soluble P-, E-, and L-selectins42 and soluble thrombomodulin.39 High levels of membrane-bound and plasma-soluble P-selectin and E-selectin have been found in ET patients with thrombosis, suggesting that sustained endothelium and platelet activation might contribute to the pathogenesis of thrombosis in these diseases.43 In addition to releasing substances that stimulate thrombus formation after injury, endothelial cells release the platelet inhibitor nitric oxide (NO), providing a negative feedback mechanism for the propagation of thrombus formation.44 NO is a free radical product generated through the oxidation of L-arginine to L-citrulline by NO synthases. The endothelial-derived NO is one of the main mediators influencing vascular hemodynamic and the interaction of leukocytes and platelets with endothelial cells. In fact, NO mediates vascular relaxation in response to vasoactive substances and shear stress; inhibits platelet adhesion, activation, secretion, and aggregation; and promotes platelet disaggregation. Moreover, NO inhibits the expression of P-selectin on platelets and impairs leukocyte adhesion to the endothelium. Clinical conditions have been reported in which a deficiency of endogenous NO production may contribute to a thrombotic event. Our group recently studied circulating NO in MPN patients42 and found reduced plasma levels of NO in patients with ET compared with controls. This confirms the previous observation that in MPN patients with thrombocytosis, the production of NO by platelets is impaired.45 However, in the same study and for the first time, we observed that ET patients treated with hydroxyurea presented with the highest levels of plasma NO. A similar effect of hydroxyurea on NO plasma levels was reported in patients with sickle cell anemia46 and may contribute to the known ability of hydroxyurea to prevent thromboembolic complications in ET patients.12 In the same study, PV patients showed high plasma NO levels compared with controls and these levels were not affected by hydroxyurea treatment. This result was as expected, because a high hematocrit level is associated with increased NO in the blood and may represent a compensatory mechanism in a high-thrombotic-risk situation.
Recent studies have shown that angiogenesis plays an important role in the biology of hematological malignancies including MPN.47 Levels of circulating endothelial cells, together with serum levels of VEGF and other proangiogenic cytokines, have been studied in MPN as markers of angiogenetic activity. The number of circulating endothelial cells (resting, activated, apoptotic, and circulating precursor endothelial cells) was repeatedly found to be increased in patients with MPN regardless of JAK2V617F status and was not affected by cytoreductive treatment.48–50
All of these lines of evidence suggest that endothelium in MPN is activated, suggesting an important endothelial contribution to the hypercoagulable state. In addition, angiogenesis may have a role in the pathophysiology of MPN.
Role of the JAK2V617F mutation
The demonstration of the acquired gain-of-function V617F mutation in the tyrosine kinase JAK-2 gene has greatly influenced the diagnostic and therapeutic approach in MPN patients. Several studies have implicated the JAK2V617F mutation in the increased thrombotic tendency observed in ET and PV patients and 3 independent meta-analyses were published recently.51–53 In the analysis by Ziakas et al involving 2905 ET patients,51 the JAK2V617F mutation was associated with an increased risk of both venous (odds ratio [OR] = 2.09; 95% CI, 1.44-3.05) and arterial thrombosis (OR = 1.96; 95% CI, 1.43-2.67), as well as thrombosis at presentation (OR = 1.88; 95% CI, 1.38-2.56). The meta-analysis by Dahabreh et al showed in 2436 ET patients that the risk of arterial (OR = 1.68; 95% CI, 1.31-2.15) and venous (OR = 2.5; 95% CI, 1.71-3.66) thromboses were significantly increased in JAK2V617F+ compared with wild-type patients.52 Similarly, the most recent meta-analysis of 21 studies involving ET patients and 6 studies with MF, showed that in ET patients, the JAK2V617F mutation is associated with a significant 2-fold increased risk of thrombosis (OR = 1.92; 95% CI, 1.45-2.53) of both venous (OR = 2.49; 95% CI, 1.71-3.61) and arterial (OR = 1.77; 95% CI, 1.29-2.43) vessels; its role in PMF patients is uncertain.53 The association between the JAK2V617F mutation and thrombosis was recently confirmed in a retrospective multicenter study from Korea involving 239 patients with ET.54 In that study, previous thrombotic history and the JAK2V617F mutation were associated with a higher 10-year cumulative incidence rate of thrombohemorrhagic events.
Although many studies and 3 meta-analysis have been published on the role of JAK2V617F mutation in the increased thrombotic tendency in ET, few data are available in PV. In one prospective study by Vannucchi et al including 173 PV patients, those subjects with a mutant allele burden > 75% had a 3.56-fold higher relative risk (95% CI, 1.47-7.1) of total thrombosis, particularly during the follow-up (relative risk = 7.1; 95% CI, 1.6-10.1).55 A retrospective analysis of patients with either PV or ET found that the frequency of thrombosis progressively increased according to the allele burden in both types of diseases, with the highest rate of vascular complications in patients with an allele burden > 50%.56 However, in a recent prospective study of patients with PV, no significant relationship between the mutant allele burden and the risk of thrombosis was observed.57
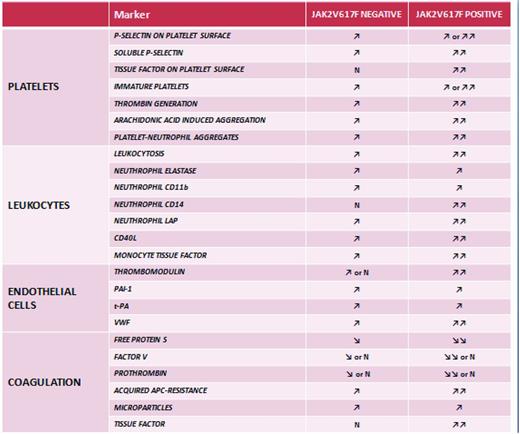
Few studies have been published addressing whether the JAK2V617F mutation may specifically affect the hemostatic system.20,39,58,59 These studies indicate that both cellular (ie, platelets and leukocytes) and plasma compartments of hemostasis were more activated in those patients positive for the JAK2V617F mutation (Figure 5). Regarding cellular components, P-selectin was found to be increased on platelets from JAK2V617F+ ET patients20 ; CD14 and LAP were demonstrated to be more highly expressed on neutrophils from ET JAK2V617F mutation carriers39 ; and a significantly higher expression of CD11b was observed on neutrophils and monocytes from JAK2V617F+ PMF patients.59 In addition, our group were able to demonstrate an elevated expression of TF in platelets and increased levels of circulating platelet/neutrophil aggregates from JAK2V617F+ ET patients compared with JAK2V617F− patients.39 The evidence that both platelets and neutrophils from JAK2V617F+ patients expressed increased activation features is in good agreement with the findings of increased mixed-cell aggregate formation in ET patients carrying the JAK2V617F mutation. Among hypercoagulation parameters, plasma levels of soluble thrombomodulin were found to be elevated in JAK2V617F+ ET patients,39 and soluble P-selectin levels were significantly elevated in JAK2V617F+ ET, PV, and PMF patients compared with JAK2V617F− patients.58 In a study by Marchetti et al, an involvement of the JAK2V617F mutation was observed in the presence of an acquired APC resistance phenotype.60
Hemostatic alterations in JAK2V617F+ and JAK2V617F− MPN patients. Abnormalities of platelets, erythrocytes, leukocytes, and endothelial cells lead to systemic hypercoagulability as represented by an increased production of procoagulant microparticles and the occurrence of an acquired APC resistance. Studies addressing whether the JAK2V617F mutation may specifically affect the hemostatic system indicate that both cellular (ie, platelets and leukocytes) and plasma compartments of hemostasis are more activated in MPN patients positive for the JAK2V617F mutation. Therefore, the expression of the JAK2V617F mutation may represent a molecular lesion relevant to promote the cellular procoagulant phenotype. N indicates not different from healthy control subjects; ↗, elevated compared with healthy control subjects; ↗↗, elevated compared with JAK2V617F− patients.
Hemostatic alterations in JAK2V617F+ and JAK2V617F− MPN patients. Abnormalities of platelets, erythrocytes, leukocytes, and endothelial cells lead to systemic hypercoagulability as represented by an increased production of procoagulant microparticles and the occurrence of an acquired APC resistance. Studies addressing whether the JAK2V617F mutation may specifically affect the hemostatic system indicate that both cellular (ie, platelets and leukocytes) and plasma compartments of hemostasis are more activated in MPN patients positive for the JAK2V617F mutation. Therefore, the expression of the JAK2V617F mutation may represent a molecular lesion relevant to promote the cellular procoagulant phenotype. N indicates not different from healthy control subjects; ↗, elevated compared with healthy control subjects; ↗↗, elevated compared with JAK2V617F− patients.
Circulating platelets are heterogeneous in size and structure. In healthy subjects, a small percentage of circulating platelets (2%) are the so-called reticulated or immature platelets recently released from the BM. Their number, reflecting the rate of thrombopoiesis, is directly correlated with megakaryocyte proliferating activity. In vitro studies show that newly formed platelets have a higher hemostatic activity compared with mature platelets, as demonstrated by the increased response to thrombin and higher expression of surface P-selectin.61
Recently, in 46 ET and 38 PV consecutive patients, we found a positive correlation between the JAK2V617F mutation and the quantity of immature platelets.25 Furthermore, we observed that the levels of circulating immature platelets are susceptible to myelosuppressive treatment, which may explain the favorable effect of hydroxyurea therapy on MPN outcome, as well as the associated thrombotic risk.
Few studies have evaluated the molecular mechanisms by which JAK2V617F mutation can affect the prothrombotic phenotype of a cell. The JAK2V617F-activating mutation can cause an increase in RBC adhesiveness through modification of surface adhesion molecules, thus facilitating thrombosis, and may render platelet hyperresponsive through altered expression of c-MPL signal transduction for TPO-induced platelet priming.62 Activation of JAK2 is also involved in TF expression by neutrophil and monocyte through the MAPK and PI3K pathway.63
Finally, the presence of JAK2V617F in both endothelial cells and hematopoietic cells belonging to BCS patients with PV has been demonstrated. This indicates that endothelial cells from these patients are involved in the malignant process and suggests that in this subpopulation of patients, the disease may originate from a cell common to the hematopoietic and endothelial cell systems.64
Other somatic mutations have been characterized in MPN, including several mutations in JAK2 exon 12 (< 5% of JAK2V617F− PV patients), and a mutation in the MPL gene (MPL W515) described in approximately 10% of PMF patients and 1% of ET patients, but not in PV patients. No information is available on the thrombophilic state of MPN patients carrying these rare mutations. The JAK2 exon 12 and MPL W515 mutations have not been detected in patients with BCS and PVT,3 whereas MPL-mutated ET has been associated with high platelet count, microvascular symptoms, and a high risk of postdiagnosis arterial thrombosis.65,66
Main prothrombotic features in the blood
In patients with ET and PV, the main prothrombotic features that manifest in the blood as a consequence of the activation of MPN clone–derived blood cells and host normal vascular cells are an increased thrombin generation sustained by an acquired APC resistance, and the appearance in the circulation of high levels of procoagulant microparticles derived from platelets and vascular cells.
Acquired APC resistance
PC, a serine protease activated by thrombin bound to the endothelial receptor thrombomodulin, is one of the major physiological anticoagulant in humans. APC, in complex with its cofactor, protein S (PS), reduces blood clotting activation through the proteolytic inactivation of coagulation factor V and factor VIII. A resistance to inactivation by APC, inherited or acquired, is associated with an increased risk of thrombosis in many situations, including pregnancy, oral contraceptive use, hormone replacement therapy, and cancer. Decreased levels of PC and PS can be responsible for the occurrence of an APC-resistant phenotype, and studies have reported a reduction in the concentration of natural anticoagulants in patients with MPN.67 By using the thrombin-generation assay, we demonstrated the occurrence of an acquired APC resistance phenotype in ET and PV patients.60 The results analyzed according to the presence (ie, positivity or negativity) and status (ie, heterozygosity or homozygosity) of the JAK2V617F mutation indicated that JAK2V617F mutation carriers are more APC resistant than noncarriers, especially if they are homozygous (ie, JAK2V617F allele burden > 50%). This suggests a progression to the APC-resistant phenotype determined by the JAK2V617F status and, together with previous observations, supports the hypothesis of a more hypercoagulable condition in JAK2V617F carriers. Prothrombin, factor V, free PS, and tissue factor pathway inhibitor levels were significantly reduced in patients and mainly in JAK2V617F carriers. Multiple regression analysis indicated that the low free PS level is a major determinant of the increased APC resistance. A high prevalence of acquired APC resistance, determined as the classical prolongation of activated partial thromboplastin time after the addition of APC, was reported by Arellano-Rodrigo et al in patients with ET6 and was found more frequently in those patients with previous history of thrombosis; again, decreased levels of free PS were detected and were inversely correlated with JAK2V617F allele burden.
Several lines of research indicate that a protease from platelets is able to cleave PS in plasma.68 On the basis of the finding of decreased PS levels in MPN, we decided to investigate the relationship between platelet-associated PS cleaving activity and in vivo PS cleavage in a group of ET patients.69 PS cleavage was found to be significantly increased in patients with ET, along with elevated levels of platelets, and returned to normal values in ET patients on hydroxyurea treatment with normal platelet counts. Therefore, proteases from platelets seem to contribute to the presence of cleaved PS in the circulation of ET patients and may enhance the coagulation response in vivo by down-regulating the anticoagulant activity of PS.
Plasma microparticles
Microparticles are membrane fragments ranging in size from 0.1-1 μm that are released by most cell types, including blood and vascular cells, upon activation. Microparticles are known to be elevated in thromboembolic diseases and malignancy,70 including patients with ET.71 The levels and cellular origin of microparticles were determined by flow cytometric analysis, and the microparticle-associated procoagulant activity was measured using a thrombin-generation assay. A significantly higher number of circulating microparticles positive for platelet and endothelial markers, as well as for TF, were found in ET subjects compared with controls. In addition, microparticle-rich plasma from patients with ET showed higher thrombin-generation potential, which was significantly correlated with the total number of microparticles. A subsequent study by Duchemin et al has shown the presence of an acquired “thrombomodulin-resistant” phenotype in PV and ET patients that is partially determined by circulating microparticles.72
Conclusions and future perspectives
Thrombotic complications significantly affect the morbidity and mortality of patients with MPN. In addition, MPN patients commonly present with abnormalities in laboratory coagulation tests that are consistent with a hypercoagulable state. The pathogenesis of blood activation in these diseases is complex and multifactorial, and involves the abnormalities of platelets, erythrocytes, and leukocytes arising from the clonal proliferation of hematopoietic progenitor cells and abnormalities of endothelial cells. These alterations include not only quantitative changes in the number of circulating blood cells, but also qualitative changes in cellular molecular characteristics. These properties lead to an increased production of procoagulant microparticles and the occurrence of an APC-resistant phenotype and very likely contribute to clotting system activation. Clinical data indicate an association of the JAK2V617F mutation with disease severity. Biological data also show an association of this mutation with the expression of cellular and soluble biomarkers of coagulation activation. However, future clinical research should focus on the evaluation of the role of these biomarkers in identifying MPN patients at higher risk of thrombosis, who may benefit from primary thromboprophylaxis. Finally, a better understanding of the molecular events leading to the development of the hypercoagulable state in Ph− MPN patients may provide appropriate tools for targeted therapies to reverse coagulopathy.
Acknowledgments
The work conducted at the Laboratory of the Division of Immunohematology and Transfusion Medicine, Ospedali Riuniti di Bergamo (Bergamo, Italy) was partially supported by grants from the Associazione Italiana per la Ricerca sul Cancro and from the National Institutes of Health Myeloproliferative Disorders Research Consortium (both to A.F.).
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Anna Falanga, MD, Division of Immunohematology and Transfusion Medicine, Ospedali Riuniti di Bergamo, Italy, Largo Barozzi, 1, 24128 Bergamo, Italy; Phone: 39-035-266578; Fax: 39-035-266659; e-mail: annafalanga@yahoo.com.