Abstract
The discovery of the JAK2V617F mutation triggered an unexpected flowering of basic and clinical studies in the field of myeloproliferative neoplasms (MPNs), resulting after just a few years in an exceptional amount of new information. One important consequence of those new findings was the modification of the World Health Organization classification and diagnostic algorithms for these diseases, which is still based on the original concept developed by William Dameshek in 1951 and keeps distinct entities under the umbrella of classical Philadelphia-negative MPNs. These MPNs are essential thrombocythemia, polycythemia vera, and primary myelofibrosis. Could a new molecular classification be a better tool to manage MPN patients? Several studies have shown that essential thrombocythemia and primary myelofibrosis can be divided into distinct subtypes based on the presence of the JAK2V617F mutation. Can we now define JAK2-positive diseases to depict a distinct entity from JAK2-negative MPNs? This chapter reviews the significance of JAK2 mutation positivity in the diagnosis, prognosis, and therapy of MPNs.
Introduction
Many changes have occurred in recent years in the field of myeloproliferative disorders, including a new World Health Organization (WHO) classification changing their name to “myeloproliferative neoplasms” (MPNs).1 Most of these significant changes resulted from the discovery of the JAK2V617F mutation, which clearly opened a new era in the understanding of MPN biology, clinical evolution, and therapy.2 This new era gives us the opportunity to reconsider many aspects of MPNs in the light of molecular lesions. JAK2V61F being the most frequent mutation in MPNs, this chapter focuses on available data challenging the robust classification proposed by William Dameshek in 1951. Dameshek identified a spectrum of diseases sharing cardinal features that were grouped under the term “myeloproliferative disorders.”3 This chapter reassesses currently used MPN classification, clinical phenotype and risks, and treatment algorithms in light of the JAK2 mutation.
JAK2 mutation as a diagnostic tool
The JAK2V617F mutation can be detected in approximately 95% of polycythemia vera (PV) patients4 ; other JAK2 mutations located in exon 12 can be found in 2%-5% of PV patients, so virtually all PV patients carry hematopoietic cells affected by an activating mutation in the JAK2 protein. Therefore, the diagnostic value of detection of the JAK2 mutation in PV is pivotal and is a key feature recognized in the most recent WHO classification.5 In this system, identification of the JAK2 mutation and erythrocytosis are the 2 major criteria allowing PV diagnosis when a minor confirmatory criterion is also present (among the following: low serum erythropoietin level, BM biopsy findings, and the presence of endogenous erythroid colony formation).
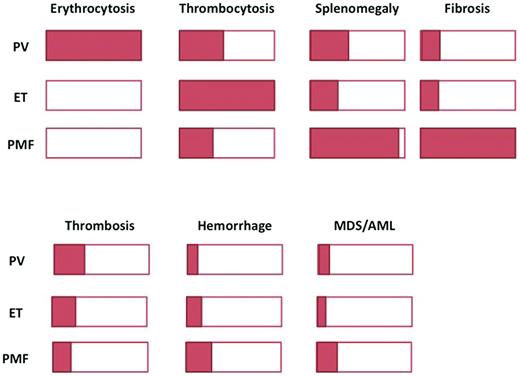
In essential thrombocythemia (ET) and primary myelofibrosis (PMF), the JAK2V617F mutation is found in approximately 50%-60% of patients (hereafter referred to as V617F-positive patients).4 Unlike PV, which is the only disease causing an increased RBC mass,6 these 2 illnesses do not display a unique clinical or biologic characteristic allowing their unequivocal identification when the JAK2 mutation is present (Figure 1). Thrombocytosis, which is a key feature of ET, can also be present in some PV or PMF patients; it is also a key diagnostic feature for refractory anemia with ringed sideroblasts associated with thrombocytosis (RARS-T), a well-defined entity classified by the WHO in the subgroup of myeloid neoplasms named “myelodysplastic/myeloproliferative neoplasm” (MDS/MPN).1 In addition, a significant proportion of patients with RARS-T carry the JAK2V617F mutation (found in 50%-80% of patients). Splenomegaly, a common finding in PMF, is also present in approximately 30% of PV and ET patients. BM biopsy morphology can discriminate between these diseases,7 but due to the subjective nature of some analyses and overlaps, its universal reproducibility has been questioned (Figure 1).8 For those diseases, the detection of the JAK2 mutation seems to be important in providing clear evidence for malignant, clonal hematopoiesis, but it must still be incorporated in a series of clinical and biologic features to allow a final MPN diagnosis.5
Overlaps in clinical presentation and evolution. Schematic representation of the proportion of patients presenting with a specific feature. The top panel shows clinical characteristics and the bottom panel, complications.
Overlaps in clinical presentation and evolution. Schematic representation of the proportion of patients presenting with a specific feature. The top panel shows clinical characteristics and the bottom panel, complications.
The JAK2V617F mutation has also been described with lower frequencies in other hematologic malignancies such as chronic myelomonocytic leukemia, in which up to 8% of patients may carry the mutation.9 Occasionally, the JAK2V617F mutation can also be found in primary acute myeloid leukemia (AML), MDS, and chronic myeloid leukemia. Some studies have also detected a few cases of low levels of the JAK2V617F mutation in the general population, but its signification in this context has to be clarified, because in many cases lack of hematologic data blunted the significance of this biologic finding.10
Considering the whole spectrum of diseases that may present with a JAK2 mutation, the only unambiguous information that one can obtain from JAK2 mutation positivity alone is that this individual patient has a myeloid malignancy. In this diagnostic perspective, JAK2 mutation testing is only another piece of the puzzle that still needs to be associated with clinical, biologic, and pathologic data to allow proper diagnosis.5
JAK2 mutation and the MPN phenotype
The finding of the JAK2 mutation in several related illnesses led to the speculation that its presence or absence or its quantity could modify the disease phenotype. In animal models, JAK2V617F expression usually leads to the development of an MPN-like disease. In transgenic models, the expression level of JAK2V617F has been shown to play an important role in determining MPN phenotype.11,12 In these models, increasing mutant JAK2 expression changes the phenotype from ET-like (in mice with a low JAK2V617F expression), to PV-like and finally myelofibrosis (MF)–like diseases in mice expressing higher levels of mutant JAK2. In knock-in models, however, heterozygous expression of JAK2V617F is sufficient to induce a PV-like disease directly.13
Ex vivo studies have provided some interesting results recently. The study of individual erythroid colonies derived from patients with PV and ET showed that JAK2V617F homozygosity apparently arises with similar frequencies in PV and ET, but expansion of a dominant homozygous subclone was found only in PV, likely due to acquisition of additional molecular lesions.14 In this model, development of a dominant JAK2V617F-homozygous subclone drives erythrocytosis in many PV patients, with alternative mechanisms operating in those with small or undetectable homozygous-mutant clones. Another study found that the thrombopoietin receptor MPL can induce antiproliferative effects in cells with high JAK activation levels, such as cells with the JAK2V617F mutation.15 These findings could explain why many PV and PMF patients with homozygous JAK2V617F show down-modulation of MPL levels in platelets, a mechanism allowing cells to escape this antiproliferative effect of MPL.
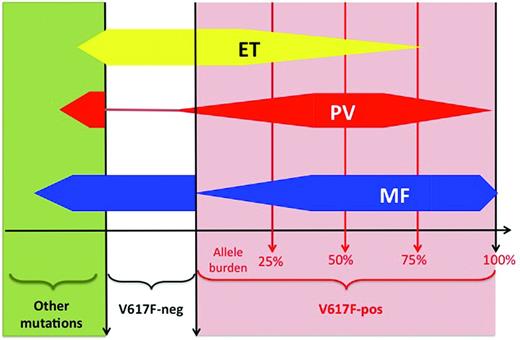
In clinical studies, many investigators quantified the JAK2V617F mutant allele, referred to as the “JAK2 mutant allele burden,” using semiquantitative PCR assays in peripheral blood–derived DNA usually extracted from purified granulocytes. Often, when the quantity of JAK2 mutant is found to be greater than 50%, the patient carries a homozygous mutation, whereas patients with less than 50% JAK2 mutant allele burden are considered heterozygous for the mutation. This shortcut is, properly speaking, incorrect because only individual cell genotyping (ie, performed in clonal colonies grown from single-cell assays in vitro) can really be assessed for their mutational status.16,17 For example, a patient carrying few homozygously mutated cells will display a low global allele burden when measured in the bulk granulocytic DNA. Therefore, using the 50% threshold in granulocytic DNA for defining homozygosity fails to detect patients carrying some homozygous clones. In addition, many different assays with different sensitivities are currently used,18 and a standardization process launched by the European LeukemiaNet (ELN) organization will hopefully result in better comparability between studies.19 With these limitations, several studies showed that JAK2V617F “homozygosity” was uncommon in ET patients and more frequent in PV and PMF patients (Figure 2).16,17 The mutant allele burden usually increases across the spectrum of these 3 classical MPNs, with the highest levels being found during fibrotic transformation.20,21 However, due to important overlaps, quantification of JAK2V617F allele burden alone does not allow discrimination between MPNs, and quantitative assays have not been shown to be clearly relevant for MPN diagnosis in standard clinical practice. In PV, in which a mutation in JAK2 is virtually always present, only some mutant JAK2 compared with wild-type JAK2 could be studied with respect to a possible influence on disease phenotype. Such studies showed that higher JAK2V617F allele burden was associated with pruritus, larger spleen size, higher cell counts, and fibrotic evolution.20
Overlaps in molecular lesions. Schematic representation of the distribution of molecular lesions across diseases. For example, in ET (yellow shape), 50% of patients have a JAK2-V617F mutation (red area, right part of the panel), whereas 50% are V617F-negative (white area) or display a mutation different from JAK2-V617F (green area, left part of the panel). Among JAK2-positive ET patients, the majority have a low allele burden (median around 20%), but a few may have a higher allele burden. In PV (red shape), the majority of patients are positive for the JAK2-V617F mutation, with a higher median allele burden (approximately 50%). In PMF, approximately 60% of patients are JAK2-V617F positive, with a high allele burden (often above 50%). Other mutations include: MPL, JAK2-exon 12, TET2, ASXL1, LNK, CBL, EZH2, SOCSs, IDHs, p53, NRAS, NF1, IKZF1, RUNX1, and RB. V617F-neg indicates the absence of the JAK2-V617F mutation; V617F-pos, the presence of the JAK2-V617F mutation.
Overlaps in molecular lesions. Schematic representation of the distribution of molecular lesions across diseases. For example, in ET (yellow shape), 50% of patients have a JAK2-V617F mutation (red area, right part of the panel), whereas 50% are V617F-negative (white area) or display a mutation different from JAK2-V617F (green area, left part of the panel). Among JAK2-positive ET patients, the majority have a low allele burden (median around 20%), but a few may have a higher allele burden. In PV (red shape), the majority of patients are positive for the JAK2-V617F mutation, with a higher median allele burden (approximately 50%). In PMF, approximately 60% of patients are JAK2-V617F positive, with a high allele burden (often above 50%). Other mutations include: MPL, JAK2-exon 12, TET2, ASXL1, LNK, CBL, EZH2, SOCSs, IDHs, p53, NRAS, NF1, IKZF1, RUNX1, and RB. V617F-neg indicates the absence of the JAK2-V617F mutation; V617F-pos, the presence of the JAK2-V617F mutation.
In addition to quantification, in ET and PMF, it was possible to assess the impact of the presence of JAK mutation because approximately half of these patients are V617F-negative (Figure 2). In ET, several studies have shown that V617F-positive patients presented with older age at diagnosis, higher hemoglobin level and leukocyte count, but lower platelet counts.20,22 In PMF, the same differences were found except for the lower platelet count.20
These observations led some investigators to suggest that V617F-positive ET, PV, and PMF were in fact diseases evolving according to a continuum model in which the 3 entities are different stages of MPN: that is, PMF would represent the accelerated phase of chronic MPN, including PV and ET, and secondary AML would be the acute phase.22–24 In this model, JAK2V617F allele burden progressively increases alongside changes in phenotype, with lower allele burden inducing isolated thrombocytosis and higher levels being accompanied by increases in hemoglobin level, leukocytosis, and splenomegaly, and ultimately with fibrotic transformation. Such a model mainly based on molecular classification overlooks the contribution of other biologic assays that can still clearly delineate ET, PV, and PMF, such as BM histopathology7 and RBC mass measurement.6,25 However, recent research has shown that the clinical heterogeneity of MPNs is probably closely linked to their genetic complexity because, in addition to JAK2V617F, many other genetic or epigenetic abnormalities were discovered in these diseases.2,4 Therefore, a molecular classification of MPNs can probably not be based on the study of one gene only. Nevertheless, one could perhaps distinguish “simple” MPNs carrying only the JAK2V617F mutation defining a disease with some degree of erythrocytosis and thrombocytosis (more frequently, ET and PV) from “complex” molecular signatures with many molecular lesions (mostly PMF; Figure 2). The latter cases could be more prone to evolve to MDS or AML, as shown by the increased frequency of molecular lesion different from JAK2V617F in “accelerated” phases of MPNs.2,4
JAK2 mutation and vascular complications
Campbell et al took advantage of the large series of 806 ET patients enrolled in the prospective PT-1 trial to study the impact of the JAK2V167F mutation.22 In this study, V617F positivity had a relatively small influence on vascular complications, because the only significant difference compared with V617F-negative patients was a higher frequency of venous thrombosis in the year preceding ET diagnosis. However, this excess of thrombosis was relatively modest in absolute numbers: 11 of 414 in V617-positive patients versus 2 of 362 in V617-negative patients. In contrast, the presence of the JAK2 mutation was not associated with an increased risk of developing thrombosis (neither arterial nor venous) after trial entry. Similar results were reported in a large retrospective study in 867 ET patients by the Italian GIMEMA group, who found that JAK2 mutation at diagnosis was not a predictor for occurrence of thrombosis during follow-up (unlike the usual indicators: age > 60 years and history of thrombosis) and to leukocytosis (> 11.3 × 109/L).24 The majority of published studies could not find a prognostic value of the JAK2 mutation at diagnosis regarding vascular events.20
In PV, several studies have assessed the role of the JAK2 mutant allele burden in the risk of vascular complications (reviewed by Vannucchi et al20 ). Conflicting results were reported, probably in part due to differences in the assays used, different sample sources (eg, DNA and RNA from peripheral blood or BM cells), and variable retrospective cohorts of patients. Therefore, ELN experts considered that, based on the currently available data, JAK2V617F allele burden should not be considered as a risk factor for initiating therapy in PV and ET until this is validated in prospective studies.26
Patients suffering from splanchnic vein thrombosis, including Budd-Chiari syndrome and portal and mesenteric vein thrombosis, represent a typical group in which JAK2V617F testing is of high clinical usefulness. Approximately half of these patients have an underlying MPN, usually masked by the development of portal hypertension after the occurrence of the thrombosis.27,28 When a JAK2 mutation is detected in this context, a diagnosis of MPN can reliably be made, because the presence of the JAK2V617F mutation has been shown to be correlated with BM biopsy findings and the presence of an endogenous erythroid colony.29 Additional investigations are needed to further classify this disease, but the main unresolved question in these patients remains the usefulness of cytoreductive therapy, especially in patients with Budd-Chiari syndrome, the most serious splanchnic vein thrombosis in which underlying MPN has been shown to be associated with more severe disease.29
JAK2 mutation, hematologic transformation, and survival
Hematologic transformation to MF, MDS, or AML are recognized long-term complications of PV and ET.23 Such evolution is indeed a matter of concern in patients who are expected to live for several decades, although the exact incidence of such transitions is still debated. They can occur spontaneously as long-term sequelae of the chronic phase or they may be favored by the use of leukemogenic therapies such as radioactive phosphorus or alkylating agents.30,31 Increasing V617F-mutant allele burden have been associated with the risk of evolution to MF, but no clear data have emerged about the risk of developing MDS/AML according to JAK2 mutational status. Conversely, JAK2V617F has been shown to induce genetic instability, to influence gene expression through modifications of chromatin structure, and to reduce apoptotic response to DNA damage, mechanisms that may increase the risk of accumulation of genetic lesions leading to acute transformation.4
Leukemic clones emerging from V617F-positive chronic MPN can be either V617F-positive or V617F-negative.32 V617F-positive AML more frequently occurs after transition to the MF phase, whereas V617F-negative AML seems to arise directly from chronic-phase JAK2-positive MPN.33 These 2 different paths to acute transformation may be related to the evolution of a unique founder clone evolving from the chronic to the acute phase after accumulation of genetic lesions or to acute transformation of an independent stem cell; this latter circumstance may be favored by leukemogenic therapies administered during the chronic phase.33
The question of a possible impact of the JAK2 mutation on overall survival of patients with MPN has not been clearly answered by previously published studies, which have provided conflicting results.20 For example, one study found that V617F-positive PMF patients had poorer survival, another found no impact, and a third showed that lower mutant allele burden was a risk factor for poorer survival.20 In the largest studied series of PMF patients (n = 345), which served as a basis for the development of the International Prognostic Scoring System (IPSS) of PMF, the presence of the mutation was not associated with either prognostic score or survival.34
JAK2 mutation and MPN therapy
The first question one may ask is whether treatment of V617F-positive MPN patients should be different from that of patients without the JAK2 mutation. The PT-1 trial showed that V617F-positive ET patients responded differently to therapy than those without the JAK2 mutation, being more sensitive to therapy with hydroxyurea but not anagrelide.22 This analysis according to JAK2 mutational status showed that one of the main differences in the outcome of patients lay in a decreased incidence of arterial thrombosis in V617F-positive patients treated with hydroxyurea compared with anagrelide (plus aspirin in both arms), a difference not found in V617F-negative patients. These results suggest that ET patients with the JAK2 mutation who are treated with conventional cytoreductive drugs gain more benefit from hydroxyurea than anagrelide therapy, whereas no significant difference in outcome was noticed between the 2 treatment arms in V617F-negative patients. Such a difference in response to therapy between V617F-positive and V617F-negative patients has not to our knowledge been clearly demonstrated in a prospective study of PV and PMF patients.
The discovery of the JAK2V617F mutation has prompted the assessment of several drugs exhibiting an inhibitory activity against the JAK family members, because it appeared to be an ideal candidate for a new era of targeted therapy in MPNs.35,36 None of the drugs currently used to target JAK2 is specific for the JAK2V617F mutant form and all of them are global JAK2 inhibitors, inhibiting mutated as well as nonmutated JAK2. This lack of specificity against the mutant form of JAK2 may be clinically useful, explaining how these drugs are also efficient in patients who do not have the JAK2V617F mutation but in whom aberrant activation of the JAK-STAT pathway is still present. For example, no impact of the JAK2 mutational status on response to a JAK inhibitor was observed in the European registration trial COMFORT II, which investigated ruxolitinib in patients with PMF.37 In that study, subgroup analyses showed similar efficacy in patients with or without the JAK2 mutation.38
If one considers that MPNs have entered the era of targeted therapy, the major objective of such therapy should be eradication of the malignant clone and ultimately increased survival, a major step toward cure. Until now, no clear effect on the mutant allele burden of patients with the JAKV617F mutation and no impact on BM fibrosis could be firmly demonstrated in ruxolitinib-treated patients. Such findings would indicate a specific effect on the MPN clone, and therefore a possible impact on disease natural history. Promising results have been reported in early-phase trials with other drugs targeting JAK2, including SAR302503 for V617F allele burden and LY2784544 with respect to BM fibrosis, or epigenetic modifiers such panobinostat, a pan-deacetylase inhibitor that induced a significant reduction in BM fibrosis in few PMF patients.35,36,39 These preliminary findings must be confirmed in larger randomized trials.
There is now evidence that all of the driver mutations lead to JAK2 activation in MPNs and that the development of erythrocytosis, thrombocytosis, and leukocytosis is related to an activation of cytokine receptor signaling. This altered cytokine receptor signaling is therefore a hallmark of MPNs and a common pathogenic feature, even in patients that do not have (yet) an identified mutation. Therefore, currently available JAK inhibitors cannot be considered strictly as targeted therapies (affecting a kinase that is also necessary for normal hematopoiesis), and responses are not expected to be different between various MPNs or diseases related to different mutations.
To date, the only drug that has demonstrated a potential curative effect in some patients with MPN remains IFN-α.40 In addition to high rates of clinical and hematologic responses, such an effect includes significant reduction in JAK2V617F allele burden comprising complete molecular responses,41,42 reversion of BM morphologic MPN-related abnormalities,43–45 and the persistence of complete responses after treatment discontinuation (up to 64 months) in some patients.45 Because IFN-α does not specifically target JAK2, it is not surprising that treatment efficacy in terms of clinical and hematologic responses is observed in MPN patients regardless of their mutational status.42 This may be different in terms of molecular responses, because complete molecular responses seem to be more frequently achieved in PV than in ET patients.42 However, although IFN-α is able to selectively eliminate JAK2V617F clones, it could be less efficient against clones bearing other mutations. For example, clones harboring TET2 or ASXL1 mutations are clearly less sensitive to IFN-α therapy. In some patients with both JAK2 and TET2 mutations, IFN-α was able to eradicate JAK2-mutated clones without affecting the TET2-mutated ones, suggesting that one drug alone, especially if targeting one pathway, may be insufficient to cure MPNs.46 Indeed, the genetic complexity of MPNs suggests that they are good candidates for personalized medicine. Ideally, simultaneous mapping of all the genetic alterations present in one individual patient could lead to propose the best medicine or the best combination of drugs able to effect cure by eliminating all of the different malignant subclones.
60 years after Dameshek, is it time to switch to a molecular classification of MPNs?
The possible relevance of defining JAK2-positive MPNs as an entity in many aspects of these illnesses has been reviewed herein. From a scientific point of view, it is obvious that past and ongoing research surrounding the effects of the JAK2 mutation in hematopoiesis is extremely stimulating and has resulted in major advances. From a clinical point of view, research in MPNs has never been so active, and many investigators have contributed to improving the management of MPN patients due to important findings related to JAK2 mutation (the author apologizes to those researchers whose work could not be cited due to space limitations).
Defining “JAK-opathies” as a homogeneous category of disorders characterized by the presence of the JAK2 mutation seems impossible: JAK2-positive MPN patients share some common features, but the WHO Dameshek-based classification seems still more relevant in clinical practice. It is still useful to properly diagnose ET and carefully exclude PV (which has a higher risk of vascular complication) and early stages of PMF (with poorer outcome) in a patient presenting with thrombocytosis. JAK2 positivity is an extremely important tool in that perspective, but currently available data do not firmly support any different management due to the presence or absence of the JAK2 mutation. However, the clinical classification of MPNs also has limitations due to some arbitrary thresholds and overlaps between clinical features (Figure 1).
Recently, many other mutations have been described in MPNs, sometimes associated with JAK2V617F, and we now have a better understanding of the genetic complexity of MPNs.2,4 The lower frequency of these non-JAK2 mutations and the lack of large prospective cohorts of patients still prevent a clear understanding of their role in disease presentation and evolution. In the near future, new techniques such as next-generation sequencing and international collaborations collecting high numbers of samples could paradoxically help to refine our comprehension of the role of the JAK2V617F mutation by clarifying these alternative molecular lesions and will hopefully provide new targets for MPN therapy.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Shire, Novartis, and Incyte and has received research funding from Novartis and Celgene. Off-label drug use: Off-label use of IFN-α in MPN.
Correspondence
J. J. Kiladjian, Centre d'Investigations Cliniques, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75010 Paris, France; Phone: 33-142499494; Fax: 33-142499398; email: jean-jacques.kiladjian@sls.aphp.fr.