Abstract
The myelodysplastic syndromes are clonal hematopoietic disorders for which hematopoietic stem cell transplantation remains the only curative therapy. The timing of transplantation, methods of disease risk stratification, patient selection, pretransplantation therapies, and preparative regimens have evolved over the years, resulting in increasing disease-free survival. In recent years, alternative donor sources have been demonstrated to be a viable alternative to traditional sibling and matched unrelated donor stem cell sources. Efforts at transplantation regimen development continue with the aim of maximizing the chances of cure with minimal toxicity and improved quality of life. Integrating new knowledge regarding disease biology will be critical to continue to improve the success of hematopoietic stem cell transplantation. Exciting areas of ongoing research that may lead to reductions in posttransplantation relapse rate include posttransplantation therapies such as DNA methyltransferase inhibitors, vaccine strategies, and donor lymphocyte infusions to enhance the GVL effect.
Introduction
In recent years, there has been a dramatic increase in the understanding of the molecular pathogenesis of myelodysplastic syndrome (MDS); however, this knowledge has yet to translate into definitive therapeutic strategies. Despite these significant advances and the development of agents that have the ability to extend survival,1 haematopoietic stem cell transplantation (HSCT) is the only curative therapy. The benefits of HSCT need to be balanced against risks of nonrelapse mortality (NRM), GVHD, and immune dysfunction. Given the lack of prospective clinical trials in this area, several issues relating to transplantation for MDS remain unresolved, including: a risk stratification approach to patient selection, the role of prior therapy, choice of conditioning, appropriate timing of transplantation, and strategies in the event of posttransplantation relapse. This review aims to summarize recent evidence as it pertains to who, when, and how to perform transplantation for MDS.
Selecting the transplantation candidate
Transplantation as a therapy for MDS has evolved over the years from being restricted to young patients with minimal comorbidities to an era in which transplantation can be considered an option for a significant proportion of patients. The advent of reduced intensity conditioning (RIC) and nonmyeloablative (NMA) regimens, along with improvements in supportive care, have been critical for this progress. In recent years, a greater understanding of factors, such as improved disease stratification and the development of the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), have allowed physicians to further refine the process. Although several issues are important, 3 main factors tend to influence the decision to offer patients HSCT for MDS: age, comorbidities, and disease stage.
Age
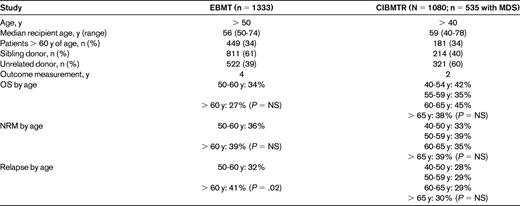
The benefit of transplantation for younger patients is generally widely accepted; greater controversy exists for older patients. Despite the median age at diagnosis being 73 years, the median age of transplantation for patients with MDS in 2010 was 55 years. From a transplantation perspective, patients more than 50 years of age can generally be considered to be “older”; however, there is a considerable lack of agreement across studies with regard to the definition of the “older patient.” Two large registry studies addressing the issue of older age have been published recently and the results are summarized in Table 1.2,3 Neither study demonstrated an adverse effect of advanced age. Given the retrospective nature of these studies, there is likely to be significant heterogeneity and inherent biases present, complicating the interpretation of outcomes. Regardless, these studies do add to a growing body of evidence suggesting that age per se should not be a contraindication to transplantation.
Comorbidities
In view of the advanced age at diagnosis of the majority of patients with MDS, comorbid conditions that affect the decision to proceed to transplantation are likely to be prevalent. Therefore, of considerable importance is finding a way of stratifying patients that allows prediction of inferior outcomes based on comorbidities. The development of the HCT-CI, although not yet evaluated in a prospective fashion, has been shown to predict NRM and overall survival (OS) in both myeloablative (MA) and RIC HSCT.4,5 This has been evaluated in the context of MDS and AML, with an increase in NRM and a decrease in OS with higher HCT-CI scores. In a retrospective analysis from our institution, 128 patients undergoing RIC HSCT with an alemtuzumab-based conditioning regimen for high-risk MDS and AML were evaluated.6 Patients with HCT-CI ≥ 3 had a high NRM of 42% at 3 years compared with 16% for those with HCT-CI = 0. In the study performed by Sorror et al,5 patients were stratified based on a combination of HCT-CI and disease risk status; patients with low disease risk and an HCT-CI of 0-2 had an NRM of 4% and 11% after NMA and MA conditioning, respectively. The corresponding OS at 2 years was 70% and 78%, respectively. In contrast, patients with intermediate-/high-risk disease and HCT-CI ≥ 3 had an NRM of 29% and 46% after NMA and MA conditioning, respectively; the OS at 2 years was 29% and 24%, respectively. This latter study demonstrates the additional information gained when considering the effect of both comorbidity and disease risk, thus illustrating the need to consider multiple factors when risk stratifying patients for transplantation. In addition to specific disease states and disease stages, there are other factors that may contribute to comorbidity. These include anemia, iron overload, recurrent infections, and immune dysregulation, all of which have the potential to be improved before HSCT. Whether the HCT-CI allows patients with such a high risk of NRM to be identified that they are unlikely to benefit from transplantation, particularly in the era of novel therapies for MDS, remains to be identified. Well-designed prospective clinical trials will be required to answer this question adequately.
Disease stage
An attempt was originally made to answer the question regarding timing of transplantation in relation to risk as stratified by International Prognostic Scoring System (IPSS) score using a Markov model.7 That study established the principle that immediate transplantation was preferred for patients with intermediate-2/high-risk disease, whereas patients with intermediate-1/low-risk disease did not gain benefit from immediate versus delayed transplantation at the time of disease progression. This model had limitations in that it applied only to MA regimens with sibling donors and to patients less than 60 years of age, and therefore was of limited applicability to the majority of patients with MDS. Furthermore, this model failed to consider the effect of important factors such as transfusion dependence on outcome. In an effort to overcome some of these limitations, Cutler et al applied a Markov model to 92 patients with de novo MDS undergoing RIC HSCT.8 The conclusion of this decision analysis was that patients 60-70 years of age with intermediate-1/low-risk disease did not gain in life years from immediate transplantation. Although it resolves some of the issues with the original study, issues regarding the use of the IPSS, exclusion of T-cell–deplete protocols, and patients with therapy-related MDS limit applicability. Furthermore, it is likely that some patients with intermediate-1/low-risk disease could be further stratified given the recent advances in molecular knowledge. To add to the discussion, de Witte demonstrated that patients receiving transplantations for low-risk disease had improved outcomes if the time from diagnosis to transplantation was shorter.9 This study included 374 patients with refractory anaemia and found a 4-year OS of 52%. Survival was improved in patients receiving transplantations at less than 1 year from diagnosis.
The development of a revised IPSS cytogenetic classification has highlighted the additional prognostic information that can be gained by refining categories based on cytogenetic risk. Application of the new scoring system10 to 1042 patients with MDS receiving transplantations at Fred Hutchinson Cancer Research Center showed 3-year OS probabilities with very good, good, intermediate, poor, and very poor cytogenetics of 46%, 46%, 41%, 37%, and 8%, respectively. In addition, the discovery of molecular abnormalities such as SF3B1, EZH2, ASXL1, RUNX1, and ETV6, while not yet studied specifically in the context of transplantation, aid prognostication and thus offer the opportunity to refine our ability to select patients for HSCT.11,12 The application of additional risk stratification via emerging diagnostic technologies such as next-generation sequencing and gene-expression profiling may also be useful.13 In one study, a flow cytometric scoring system showed a correlation between that score and the risk of relapse after transplantation.14 Whereas the controversy regarding who benefits the most from transplantation. especially in patients with lower-risk disease, is far from resolved, it is hoped that further refinement of diagnosis, application of scoring systems, consideration of comorbidities, and better risk stratification based on immunophenotypic or molecular methods may resolve these issues in the future. Ultimately, the decision on whom and when to transplant must remain an individual patient–focused decision based upon available evidence. This needs to occur with an understanding of the complexities of MDS, accurate assessment of patient comorbidities, effect on patient quality of life, and acknowledgement of the limitations of the current evidence base.
Bridge to transplantation: conventional chemotherapy
An increasing body of evidence indicates that a high pretransplantation disease burden is correlated with poor posttransplantation outcome.15,16 In the Criant study, intensive antileukemic therapy before transplantation to induce complete remission (CR) resulted in an encouraging 4-year OS of 54%.17 In the donor versus no donor comparison, the benefit of allogeneic HSCT was greatest in patients with intermediate- or high-risk cytogenetics. Interestingly, in that study, patients with no donor who received either further consolidation or autologous transplantation had a 4-year OS of 27% and 37%, respectively, suggesting that some patients may achieve sustained remissions with intensive chemotherapy alone. In an analysis of 84 patients receiving HSCT for MDS, Warlick et al demonstrated that patients with > 5% blasts at the time of transplantation had a 1-year relapse rate of 35% compared with 18% for those who were in CR.15 Despite the evidence indicating the importance of low pretransplantation disease burden, the value of disease bulk reduction before transplantation remains to be established. In the Warlick study, pretreatment did not appear to effect relapse rates after transplantation; however, there was a trend toward improved disease-free survival (DFS) in patients receiving NMA regimens if they received pretransplantation therapy to achieve < 5% blasts. In a study of patients receiving MA transplantation, Scott et al failed to demonstrate a difference in outcomes for patients who were pretreated compared with those who were not.18 Unfortunately, most of these analyses are subject to bias because it is likely that patients who were selected to receive intensive chemotherapy before HSCT had important disease-variable differences from those who did not.
Bridge to transplantation: hypomethylating agents
The development of hypomethylating agents as a treatment for MDS has changed the treatment paradigm for many patients. For those unable to tolerate intensive chemotherapy but who require treatment before HSCT, these drugs can induce remissions and thus enable patients to receive a potentially curative option for which they may not have otherwise been eligible. The use of azacitidine and decitabine has been demonstrated to be feasible in several retrospective studies and prospective studies are in progress.19–21 Whether these agents offer an added advantage over more intensive chemotherapy remains to be established. Some investigators have postulated that these therapies may enhance a GVL effect via alteration of antigen recognition. The effect on GVHD has not been established, although previous studies have not documented an increase in the development of GVHD after transplantation. Studies from our institution suggest that 5-azacytidine induces profound immunological changes, particularly with respect to changes in T-regulatory and Th-17+ CD4 cell lineages. Given the prolonged time to response with hypomethylating agents, adequate time for a suitable donor search is possible; however, a potential disadvantage is that time to transplantation may be delayed compared with conventional chemotherapy.
In the absence of prospective clinical trials, our approach at Kings College Hospital is to proceed to transplantation with < 5% blasts whenever possible. This is more likely to be important for NMA and RIC transplantation compared with MA regimens. The method through which this is achieved depends on the interplay of patient and disease factors, including disease burden, cytogenetic risk profile, and the patient's ability to tolerate therapy.
Does iron overload matter?
Another controversial issue is the importance of pretransplantation iron burden and requirement for pretransplantation iron chelation. Several investigators have found that higher ferritin levels before HSCT are correlated with adverse outcomes. The effect of elevated ferritin is most established for MA protocols, but it has also been observed in the RIC setting.22 Although chelation improves ferritin levels, no study has yet demonstrated that chelation results in improved transplantation outcomes. Ferritin levels are a crude method of measuring iron overload. Prospective clinical trials that include additional measures such as MRI techniques to assess tissue burden and measurement of labile plasma iron will be required to determine the true impact of iron overload in the transplantation setting and whether there is any role for pretransplantation chelation.
The role of alternative donors
In recent years, it has been established that outcomes with well-matched unrelated donors are equal to that of sibling donors. A more complicated issue is the question of the best approach in the absence of such a donor. Both haploidentical and cord blood have been studied as the stem cell source, but there are relatively limited data available. Results of recent studies23–25 as they pertain to MDS are summarized in Table 2. In one of the few comparative studies, Majhail et al compared the outcomes of umbilical cord blood transplantation (UBCT) with those after sibling donor transplantation in 98 patients with MDS/AML more than 55 years of age who received a RIC protocol.23 Sixty patients had UCB (85% were double cord transplants) donations and 38 patients had a matched sibling donor. No significant differences in outcomes were reported (OS at 3 years was 37% vs 31%), leading to the conclusion that UBCT is a suitable alternative to sibling donor transplantation.
Outcomes of recent studies of UBCT in adult patients with MDS

DFS indicates disease-free survival.
There are less data available on patients receiving haploidentical transplantations for MDS. Potential advantages of this donor source include immediate availability, lower cost compared with UBCT, and availability of donor lymphocytes for immune therapy in the event of relapse. A recent trial found comparable clinical outcomes for the 2 donor sources.26 Chen et al reported on 36 patients who received transplantations from HLA-mismatched family donors using a protocol that combined G-CSF–primed BM and G-CSF–mobilized peripheral blood stem cells.27 Conditioning involved cytarabine, busulfan, cyclophosphamide, and antithymocyte globulin (ATG). All patients engrafted and the 2-year leukemia free survival was 65%. In a study of RIC haploidentical transplantation, 28 patients received a combination of fludarabine, melphalan, thiotepa, and ATG. Of 22 patients with AML/MDS, 12 had < 15% blasts at the time of transplantation; of these, 42% achieved long-term remission compared with 0% (P = .03) of those with higher disease burden at the time of transplantation.28 There is increasing interest in the use of posttransplantation cyclophosphamide as pioneered by the Hopkins/Seattle groups.29 To date, small numbers of patients with MDS have received transplantations with this approach and further data are awaited. Our own institutional experience in this setting includes 9 patients with MDS/AML. Early experience is promising, with only 1 failed engraftment, 1 relapse at 1-year after transplantation, no NRM, and no chronic GVHD.
Overall, early data for both UCBT and haploidentical transplantation for MDS indicate that these donors are a suitable alternative stem cell source, thus expanding the donor pool for patients who require HSCT.
Protocol selection: issues of intensity and T-cell depletion
With the development of NMA and RIC protocols, there now exists a large variety of different conditioning regimens, which complicates comparison and protocol selection. An illustration of this is provided in Figure 1. The advantages of RIC in terms of decreased NRM are offset by increased rates of relapse, resulting in similar overall outcomes compared with MA approaches. A recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis compared the outcomes of 3731 MA and 1448 RIC/NMA transplantation patients reported to the CIBMTR between 1997 and 2004.30 No difference in outcome was demonstrated for MA and RIC protocols; however, lower OS and DFS were reported for NMA regimens. In a subanalysis of patients, 40-60 were deemed eligible to receive MA, RIC, or NMA protocols and inferior DFS was confirmed for the NMA approach. These results raise interesting questions about the importance of regimen intensity; however, strong conclusions are hard to draw given the likely heterogeneity of the cohort and bias present when selecting patients for NMA protocols. Prospective clinical trials such as the current randomized RIC versus MA trials open in both Europe and the United States (NCT01339910 and NCT01203228) are an important step forward in efforts to answer questions regarding optimal conditioning intensity.
Diagram to illustrate intensities of commonly used regimens for MDS HSCT.
Diagram to illustrate intensities of commonly used regimens for MDS HSCT.
Successful outcomes are demonstrated for both T cell–replete and T cell–deplete protocols, with the latter having lower rates of cGVHD.31,32 This represents a significant advantage in terms of patient quality of life, with wide agreement among transplantation physicians that the effects of moderate to severe chronic GVHD are devastating. The potential disadvantage in this setting is a decreased GVL effect resulting in higher relapse rates. Whereas the issue of relapse is important, it can be argued that the T cell–deplete protocol provides an immunotherapeutic platform on which to base further therapies such as DLI and vaccine strategies. No randomized prospective trial exists comparing these modalities, and choice of approach will tend to reflect local policy and center expertise. Newer protocols have included the development of intravenous and targeted dose busulfan and treosulfan and clofarabine.33,34 One approach for high-risk patients or those not in remission has been the use of combined chemotherapy and RIC protocols. A recent study reported on the use of clofarabine-based chemotherapy followed by RIC HSCT for high-risk, relapsed, or refractory AML or MDS.35 A total of 27 patients were evaluated, with a reported 2-year OS and DFS of 56% and 52%, respectively. An interesting approach reported by Pagel et al explored the use of an iodine-radioconjugated Ab in combination with a fludarabine low-dose total body irradiation protocol.36 Ultimately, the best approach for conditioning is one that achieves the required dose intensity to prevent relapse while minimizing toxicity. There is likely to be no single protocol that is suitable given the heterogeneity of this patient population, and decisions about protocol selection should be made on the basis of individual patients' disease risk profiles and comorbidity status.
Strategies to combat posttransplantation relapse
The major limitation to the success of HSCT for MDS is the problem of relapse. To date, no definitive solution has been identified, but several promising strategies continue to be evaluated. Approaches to the management of this difficult problem include preventative strategies for those considered at high risk and preemptive strategies for those with evidence of minimal residual disease or decreasing donor chimerism. Once relapse is established, the chances of achieving sustained remissions are low and are more likely to be successful if the time from HSCT to relapse was prolonged.
Role of hypomethylating agents
The choice of pharmacological therapy for relapse after HSCT can be difficult. Reinduction chemotherapy offers a potential of obtaining a remission, but does not appear to result in long-term DFS. Furthermore, patients already compromised by previous treatment and complications after HSCT may not be able to tolerate intensive chemotherapy. This raises the question of whether some of the newer agents may be as effective (and less toxic) in this context after transplantation. Azacitidine has been evaluated recently as a possible strategy for both preventing and treating relapse. De Lima et al published the results of a dose-finding study in 45 patients at high risk after HSCT, 67% of whom were not in remission.37 This study identified 32 mg/m2 as being the optimal dose administered for 4 cycles. The 1-year OS and event-free survival were reported as 77% and 58%, respectively, and patients in CR at the time of transplantation derived the best event-free survival with this approach. A randomized prospective trial is ongoing (NCT00887068). Platzbecker et al evaluated the use of azacitidine administered in the event of decreasing CD34+ chimerism.38 In this analysis, 20 of 59 prospectively screened patients were administered 4 azacitidine cycles (75 mg/m2 for 7 days) for CD34+ chimerism < 80%. Sixteen patients initially responded in terms of increasing chimerism or stabilization in the absence of relapse. Ultimately, 13 patients went on to relapse and the investigators concluded that azacitidine used preemptively could prevent or delay hematologic relapse. A recent publication on the use of azacitidine after transplantation evaluated TREG populations and CD8+ responses to tumor antigens, with results suggesting a possible augmented GVL effect but no increase in GVHD.39 These studies raise interesting questions, but the results of prospective randomized trials will be needed to determine the efficacy of these agents after transplantation.
DLI: a potential strategy to prevent and treat relapse
There are limited published studies addressing the role of donor lymphocyte infusions (DLIs) in the context of MDS. Recently, the results of a study combining lymphodepleting chemotherapy followed by DLI were published.40 A total of 35 patients, 22 of which had AML or MDS/MPD, received DLI and 49% achieved CR. Of patients with CR, the 1- and 2-year OS was estimated at 44% and 28%, respectively—significantly better than for those who did not achieve CR. Our center has collated results on 113 patients who received DLI in the context of T cell–deplete transplantation for MDS and AML secondary to MDS.41 These data show excellent long-term survival with the use of preemptive DLI (5-year OS after DLI of 80%) given for low or decreasing donor CD3 chimerism. Patients who received DLI for relapsed disease also had good long-term outcomes with 5-year OS after DLI of 40%. Our results also indicated much better outcomes if remission was achieved before the administration of DLI. Current institutional policy is to administer DLI for donor CD3 chimerism < 50% or decreasing > 20% in 1 month at a starting dose of 5 × 105/kg (unrelated donors) or 1 × 106/kg (related donors). In the event of relapse, reinduction chemotherapy is administered first and then DLI is given if remission is achieved.
Other immunotherapeutic strategies
One attractive method of potentially enhancing the GVL effect after HSCT is via the use of leukemia vaccines. Peptide vaccination with leukemia-associated antigens such as WT1 has been evaluated in small numbers of patients with demonstrated immunological responses and some clinical responses.42,43 The use of whole-cell leukemia vaccination with CD80 and IL-2 genetically modified leukemic blasts is being explored in a phase 1 trial in our institution.44 Gamma-delta T cells have been demonstrated to have antitumor activity across a wide range of tumor types, including AML.45 Studies assessing the potential role of these cells as immunotherapy are currently in development by our group.
Conclusion
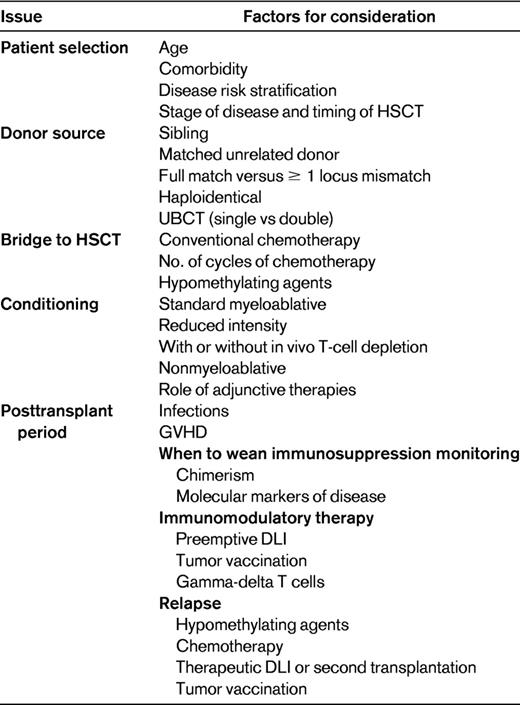
Although a minority of patients may obtain long-term remissions with intensive chemotherapy,17 transplantation for MDS has come of age and for the foreseeable future remains the only treatment modality with true curative potential. The current challenge for transplantation physicians is to achieve cure with low toxicity and maintenance of quality of life. Table 3 lists factors involved in the consideration of HSCT. Future strategies will need to involve integrating new knowledge of disease pathogenesis and targeted therapies into the transplantation process. Posttransplantation manipulation with immune-mediated strategies is an exciting area for future development.
Disclosures
Conflict-of-interest disclosure: G.J.M. is on the board of directors or an advisory committee for Celgene and Amgen, has received research funding from and consulted for Celgene, and has been affiliated with the speakers' bureaus for Celgene and Novartis. V.P. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Prof G. J. Mufti, Department of Haematological Medicine, King's College Hospital, Denmark Hill, London SE5 9RS, United Kingdom; Phone: +44-203-299-3080; Fax: +44-203-299-4980; e-mail: ghulam.mufti@kcl.ac.uk.