Abstract
Adult acute lymphoblastic leukemia (ALL) is a heterogeneous disease affected by many patient- and disease-related factors, including age, immunologic subtype, and clinical, genetic, and molecular features. Allogeneic hematopoietic cell transplantation (HCT) has occupied an increasing therapeutic role as a result of significant improvements in supportive care and histocompatibility testing. ALL Philadelphia chromosome–negative patients formerly excluded now are considered HCT candidates and survival rates with alternative donors may approach those obtained with matched-related donors. Reduced-intensity conditioning rather than myeloablative conditioning appears to provide comparable patient outcome results although these observations have not been validated in prospective studies. Improved tools can identify patients thought to be in remission based on morphology but who have active disease at the molecular or immunophenotypic level (minimal residual disease). Using B-cell antigen panels, clone-specific immunoglobulins, or T-cell receptor rearrangements to detect positivity at thresholds of at least 1 in 104 cells, such patients may be taken to HCT. The ongoing advances in conventional therapy intensity, however, now yield improved results and ongoing reassessment of the place of HCT needs to be continued; every effort should be made to enroll eligible patients in clinical trials.
Introduction and perspective
Adult acute lymphoblastic leukemia (ALL) is a relatively uncommon malignancy, accounting for approximately 20% of new acute leukemias. In 2012 in the United States, approximately 6050 new cases were diagnosed, accounting for 1440 deaths (for adults and children).1 In contrast to children, in whom the rate of cure approaches 90%, less than half of adults attain disease eradication, even with hematopoietic stem cell transplantation (HCT). In recent years, significant advances in biology have yielded valuable immunologic, cytogenetic, and genetic information that have enabled practitioners to address the marked heterogeneity and variable prognosis of ALL. In a separate chapter, Dr Charles Mullighan discusses several of these advances with an emphasis on the molecular genetic makeup of ALL. One major distinction is the presence or absence of the so-called Philadelphia chromosome (Ph) that separates patients into groups with very different clinical behaviors. This chapter will not discuss the changing paradigms for the treatment of the Ph+ subtype. Other factors present at diagnosis and the kinetics of response to therapy (eg, when morphologic complete remission has been attained and the presence of minimal residual disease [MRD]), have facilitated the development of risk-specific treatment. As a result, practitioners have begun to optimize the approach in various patient subsets. However, the treatment regimens for ALL are extremely complex, so there have been fewer randomized controlled clinical trials in ALL than in other leukemias. Despite such obstacles, randomized and nonrandomized trials and excellent observational database studies have led to novel treatment strategies, including the use of dose-intensification established in pediatric protocols, new targeted therapies, and the expanding use of reduced-intensity conditioning (RIC) HCT. Many patient- and disease-related factors influence the use and anticipated outcome in HCT. This review addresses the “when, how, and what cell source” to be considered when contemplating HCT for Ph− ALL patients in first complete remission (CR1). One major area of controversy that will not be addressed in this review is the higher chance for cure with chemotherapy in adolescents and young adults compared with older patients as a result of the use of “pediatric-like” very intensive regimens with greater reliance upon L-asparagine, dexamethasone, and other agents. Although the lower age limit for adult HCT varies considerably, for the purposes of this review, we will restrict our analysis to those subjects age 35 years and older.
Allogeneic HCT for ALL first was reported in 1973.2 Weiden et al3 noted an allogeneic effect of the infused donor cells against recipient ALL, often referred to as the GVL effect. Subsequent improvements in cytotoxic therapy and supportive care coupled with a marked expansion in the donor pool as a result of greater understanding of donor-recipient histocompatibility testing, including the use of high-resolution DNA matching for HLA-A, HLA-B, HLA-C, and HLA-DRB1 alleles (8/8 match), resulted in higher rates of survival.4
Unfortunately, most adult ALL patients who achieve CR eventually relapse. In contrast to acute myeloid leukemia, the prognosis for ALL patients who relapse is extremely poor and few are rescued even when given HCT. Fielding et al5 reported on the outcomes of 609 adult ALL patients who relapsed; overall survival (OS) after relapse was only 7%, although the prognosis was slightly better in patients younger than age 20 years and those with a prior CR1 duration of more than 2 years. Waiting beyond first CR1 to perform HCT may be problematic, because many patients will never achieve CR2. Prevention of recurrence clearly is the best strategy for long-term survival in this disease.
Standard- versus high-risk ALL
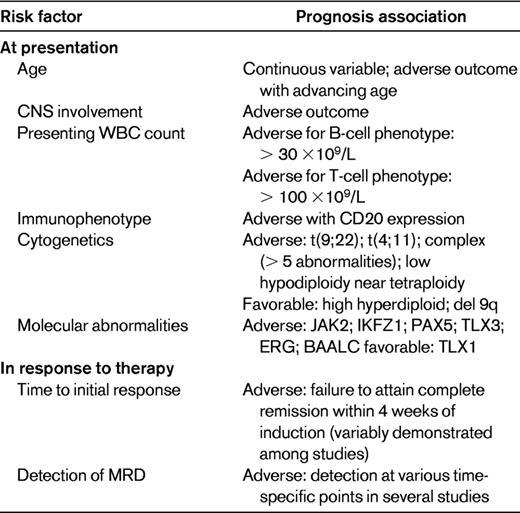
The inherent heterogeneity of ALL requires that treatment decisions reflect an accurate assignment of risk. One of the strongest adverse prognostic features is the presence of the Ph chromosome t(9;22). Rowe reviewed the prognostic clinical features at diagnosis of ALL, which are presented here in modified fashion (Table 1).6 Response to therapy, including assessment of MRD (discussed in “MRD”), is also used to risk-stratify patients.
Other high-risk situations
In addition to the Ph chromosome, Moorman et al7 identified more than 20 pretreatment specific chromosomal abnormalities associated with poor outcome. Patients with t(4;11)(q21;q23), complex karyotype (5 or more chromosomal abnormalities), or low hypodiploidy/near triploidy all had inferior rates of event-free survival (EFS) and OS compared with other patients. In contrast, patients with high hyperdiploidy or a del(9p) had a significantly improved outcome. Poor-risk cytogenetic features confer an increased risk of treatment failure and should be used to risk-stratify adult ALL. Although it has not been demonstrated that allogeneic HCT improves outcome in t(4;11) patients, this issue currently is under study.
Postremission therapies: matched-sibling HCT versus chemotherapy or autologous HCT
Four recent major prospective studies have reported patient outcomes after variable postremission therapies, with or without a family donor, who were believed to be able to tolerate allogeneic HCT8–11 (Table 2). These complex studies compared matched-sibling HCT versus chemotherapy or autologous HCT. Ribero et al8 analyzed 183 Ph− ALL patients in CR1 (eligibility age, 15-50 years) and failed to demonstrate that having a family donor available for allogeneic HCT provided superior outcomes compared with chemotherapy or autologous HCT. The reported relapse rates of 62% at 5 years in the donor group, however, were inexplicably high. Goldstone et al9 reported the MRC UKALLXII/ECOG 2993 study. The trial enrolled patients age 15-64 years, but 59 years was the upper age limit for allogeneic HCT recipients. Using a donor versus no-donor analysis, they showed that Ph− ALL patients with a donor had an improved 5-year OS (53% vs 45%, P = .01) and an associated significantly lower relapse rate (P < .001). The survival difference was significant in standard-risk patients, whereas in high-risk (especially older) patients, the reduced relapse risk was offset by an increase in nonrelapse mortality (NRM). Subjects randomized to chemotherapy had a better 5-year OS (46%) than those randomized to autologous HCT (37%, P = .03) demonstrating no benefit for a single autologous HCT. Cornelissen et al for Hemato-Oncologie voor Volwassenen Nederland (HOVON)10 in 2 consecutive, prospective studies of myeloablative (MA) HCT in ALL CR1 patients ages 15-55 years. They showed significantly better 5-year disease-free survival (DFS) in the donor group, 60% versus 42%, P = .01. As anticipated, NRM was significantly higher in the donor group (16% versus 3%, P = .002) but those patients with a donor had a markedly lower incidence of relapse at 5 years (24% vs 55%, P < .001). Again, the benefit was most pronounced in the standard-risk group, although poor-risk patients in the donor group also had a significantly improved outcome. Kako et al for the Japan Adult Leukemia Study Group (JALSG)11 developed a decision tree analysis based on the results of 2 JALSG studies (ALL93 and ALL97) to identify the optimal strategy of postremission therapy in Ph− ALL CR1 patients with a HLA-matched sibling in patients ages 15-54 years. Allogeneic HCT was superior to other therapies for 10-year survival probability: 48.3% versus 32.6%, respectively. Ram et al12 conducted a meta-analysis of previous randomized trials in ALL patients. All-cause mortality was reduced significantly (relative risk = 0.88; 95% confidence interval, 0.8-0.97) using allogeneic HCT compared with autologous HCT or chemotherapy.
Published trials in adult ALL that included > 100 patients and assignment to HCT
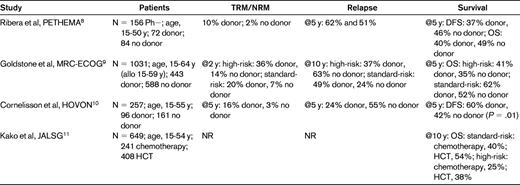
NR indicates not reported; PETHEMA, Programa Español de Tratamiento en Hematología; MRC, Medical Research Council of the United Kingdom; and ECOG, Eastern Cooperative Oncology Group.
Overall, these studies demonstrate that allogeneic HCT is superior to chemotherapy or autologous HCT for Ph− ALL CR1 patients. The statistically significant survival advantage, however, appears to be greater for patients with standard-risk rather than high-risk ALL patients. The 4 recent major randomized trials8–11 all restricted allogeneic HCT to patients age 15-59 years. Using age as a high-risk feature accounts for much of the data showing that the standard-risk group benefited the most. This information is opposite to that observed in the older randomized trials and a meta-analysis in which high-risk, not standard-risk, patients benefited from allogeneic HCT,13 a finding that likely reflects the increased treatment-related mortality (TRM) in the high-risk group that negated the GVL effect in these patients. Inclusion of the younger patients in the 4 recent randomized trials, however, may have biased the results in favor of allogeneic HCT.
URD HCT versus chemotherapy or autologous HCT
Only approximately 25% of patients have a fully histocompatible-related donor, meaning that the majority of HCT recipients will rely upon alternative graft sources including the use of MRDs, URDs, umbilical cord blood (UCB) grafts, and haploidentical donors. Which donor to use has become an increasingly complex decision process.
Nishiwaki et al retrospectively compared related and URDs as a graft source for 1139 Ph− ALL patients, 641 (310 related; 331 URD) of whom underwent HCT in CR1.14 The OS rate results of URDs approached those for related donors (62% versus 65% at 4 years, respectively, P = .19). Interestingly, at 4 years, relapse rates were significantly higher in the related group (32% vs 22%, P = .03), but the benefit was offset by significantly higher 4-year NRM: 14% versus 27% (P = .0002). Marks et al15 reported a retrospective observational database analysis of 169 matched-unrelated donor (MUD) MA allografts. At 5 years, TRM was 42%, relapse rate 20%, and OS 39%. In high-risk or recurrent ALL patients, Bishop et al16 compared autologous (n = 101) versus URD HCT (n = 159) for CR1; 64 patients received autografts and 76 underwent URD HCT. Relapse at 3 years was significantly lower for the URD group (15% vs 45%, P = .0001) but, due to high TRM, leukemia-free survival (LFS) did not differ significantly at 37% for URD and 39% for autologous HCT. These retrospective data show that for some ALL CR1 patients who do not have an HLA-matched sibling donor, an autologous HCT may be a viable option and may shorten the period of continued therapy. These HCTs, however, were performed before 1998, and with significantly improved methods of donor-recipient matching, it is very likely that the URD results would have been better. Logically, one should recommend autologous HCT only for patients unable or unwilling to tolerate chemotherapy, although, practically speaking, this scenario will be quite rare.
Other donor considerations
Degree of HLA match and donor characteristics including age, sex, parity status, race, ABO compatibility, and CMV serologic status are important. Kollman et al for the National Marrow Donor Program (NMDP)17 published a report more than a decade ago that now is being updated. They demonstrated, as had others, that chronic GVHD rates were higher with multiparous female donors compared with males (54% vs 44%, P < .0001). The use of younger donors was associated with a lower incidence of GVHD and improved survival for both HLA-matched and HLA-mismatched HCT. These URD data indicated that donor age had a greater effect on overall mortality than the other factors. Such findings pose the possibility of using a younger MUD in lieu of a fully matched-related sibling.
UCB grafts (Table 3)
Results using UCB grafts compared with alternative donor graft sources in ALL

EBMT indicates European Group for Blood and Marrow Transplantation; ENR, Eurocord-Netcord Registry; NCBP, National Cord Blood Program; JMDP, Japan Marrow Donor Program; JCBBN, Japan Cord Blood Bank Network; GETH, Grupo Español de Trasplante Hematopoyético; PETHEMA, Programa Español de Tratamiento en Hematología; UMN, University of Minnesota; and PB, peripheral blood.
UCB has emerged as a major, untapped source for HCT, providing clinicians and patients with increased choices for alternative donors. Eapen et al for the Center for Blood and Marrow Transplant Research (CIBMTR)18 retrospectively evaluated UCB versus URD grafts. The use of less well-matched UCB grafts resulted in LFS rates comparable to 8/8 and 7/8 URD allele-matched grafts. Atsuta et al compared the use of single UCB grafts with URD BM grafts and observed comparable patient outcomes.19 They more recently examined the use of single UCB grafts versus HLA-mismatched URD BM grafts.20 The single UCB grafts were associated with slower neutrophil recovery, but the incidences of acute GVHD and TRM were significantly lower; relapse rates and OS rates at 3 years were nearly identical. Ferrá et al reported 5-year relapse-free survival (RFS), DFS, and OS data; the longer follow-up times and the inclusion of a higher-risk population likely explains the lower patient outcome rates.21 Bachanova et al reported a small series of 18 adult UCB recipients (along with 4 sibling-matched blood allografts); only 12 were ALL in CR1 and 14 were Ph+.22 Outcomes at 3 years were: TRM 28%, relapse 33%, and OS 49% with a RIC regimen (see “The emerging role of RIC”). These data showed that UCB is a graft source worthy of consideration in the alternative donor HCT setting. Further, the expanding use of double UCB grafts may shorten the duration of severe neutropenia and improve relapse rates, enhancing the appeal of this product. Because many of these data were generated from observational databases, single institution studies, and studies with small patient sample sizes, caution must be exercised when interpreting these findings.
Haploidentical related donors
Valcárcel et al for the CIBMTR reported outcomes for acute leukemia patients (ALL as well as acute myeloid leukemia) in CR1 or CR2 patients treated with 1 antigen–mismatched related donor (MMRD) grafts as assessed by low-resolution HLA typing at 3 loci (HLA-A, HLA-B, and HLA-DRB1) versus MUD transplantations determined by high-resolution HLA typing at 4 loci (HLA-A, HLA-B, HLA-C, and HLA-DRB1).23 Although the incidence of chronic GVHD was significantly lower with MMRDs (P < .01), patient outcomes using MUDs were similar. In the era of high-resolution typing, however, these relatively few ALL patients and the lack of high-resolution HLA typing preclude drawing conclusions regarding the choice of a MMRD over a MUD.24 Finally, a remaining potential alternative graft source is the use of haploidentical donors for HCT. This approach remains investigational, but has shown promise when used in conjunction with posttransplantation cyclophosphamide in very-high-risk and late-stage disease patients.25,26 There are insufficient data in adults at this time to conclude whether this hematopoietic cell source is an effective treatment option. The use of alternative donors has expanded the opportunity for more patients to undergo allogeneic HCT significantly. In most instances, the results with MUD and UCB grafts now may approach the results obtained with MRDs.
The emerging role of RIC
The total body irradiation (TBI)–containing MA regimen developed at Stanford by Karl Blume et al has been used by some as a standard for conditioning, because this approach was associated with extremely good outcomes. However, there are no randomized, controlled trials examining regimen intensity in ALL, and an optimal strategy does not exist. RIC transplantation has emerged as an option designed to lower TRM and extend the HCT option to older patients and those with preexisting comorbid conditions who would not otherwise be considered viable HCT candidates.27 This approach relies heavily upon the grafted cells to exert a potentially powerful allogeneic or GVL effect. Other theoretical benefits of RIC are preservation of thymic-dependent T-cell reconstitution and shorter neutropenic periods that result in lower likelihood of bacterial infection in the peri-HCT period. MA conditioning induces significant tissue damage and a resultant “cytokine storm” that may sometimes be fatal. In addition, the use of RIC often leads to lower GVHD rates. The data supporting the use of RIC in ALL, however, are limited and difficult to fully evaluate because of regimen variability and small sample size. Table 4 shows recent trials that have reported this strategy. Stein et al,28 Bachanova et al,22 and Cho et al29 reported small, single-institution studies on 21, 22, and 37 patients, respectively. Only approximately half the patients were in CR1. These T cell–replete regimen reports had acceptable rates of acute and chronic GVHD and demonstrated RIC to be a feasible option. Nishiwaki et al30 reported a somewhat larger series with similar results. Mohty et al31 and Marks et al32 reported large observational database retrospective analyses in which they compared RIC outcomes with MA conditioning results. The report by Mohty et al31 analyzed ALL patients in CR who received HLA-identical sibling allografts, but included Ph+ ALL patients; further, the full-intensity-conditioning group had a 6% better 2-year LFS, but the study was not powered to detect small differences. The Marks et al32 study included both MUDs and sibling-matched allografts, but only Ph− patients were reported. As expected, the RIC population was older. TRM was higher for the MA group, but relapse rates were lower. LFS and OS results, however, were comparable.
Use of RIC in ALL
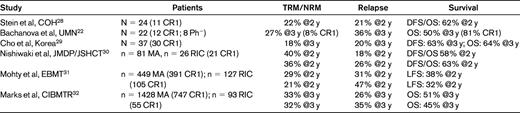
COH indicates City of Hope; UMN, University of Minnesota; JMDP, Japan Marrow Donor Program; JSHCT, Japan Society for Hematopoietic Cell Transplantation; and EBMT, European Group for Blood and Marrow Transplantation.
These studies have shown that RIC HCTs are feasible and associated with comparable donor engraftment rates and lower incidences of TRM, but with higher relapse rates than myeloablation; OS rates are similar to MA conditioning. Given the negative inherent patient selection criteria bias and the heterogeneity of RIC regimens, the less-intensive approach could actually prove to be superior. Table 4 illustrates that most of these data have been generated either from observational databases or small patient series, rather than from prospective comparison studies, and care must therefore be taken when interpreting these data. While we wait for randomized study results, ALL patients in CR1 who are at higher risk for TRM (eg, those of advanced age or with comorbid conditions) should be considered for a clinical trial; in the absence of such, RIC HCT can be considered a reasonable option. Subjects without such features but who are at higher risk for relapse should be considered for MA procedures; the data appear to show a greater benefit for use of a TBI rather than a non-TBI conditioning, especially if the dose is at least 13 Gy.32,33
MRD
Clinicians continue to rely upon morphologic means to document remission. So-called standard-risk patients in actuality could be high-risk if very low levels of leukemic cells were detected after induction chemotherapy; patients with MRD have worse outcomes. Based initially on exciting pediatric data,34 several groups have used different methods to assess MRD as a predictor for the likelihood of relapse. Leukemia-associated immunophenotype (flow cytometry) and molecular targets appear to provide equal answers with respect to MRD, but fewer patients have the latter available compared with the former. Further, the clinically most relevant time points and thresholds are still under investigation and discussion. Brüggemann for the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL)35 used real-time quantitative PCR for patient-specific Ig heavy chain/TCR rearrangement studies. They demonstrated that serial quantification of MRD identified likelihood of early relapse in 196 standard-risk patients. Over 9 time points in the first year after induction therapy, MRD information identified low-, intermediate-, and high-risk groups based on ALL burden and time of analysis, thus facilitating an individualized treatment. This group36 further provided a consensus statement on methodologies and definitions of MRD response and failure that is useful for clinical practice. Bassan et al37 obtained relevant probes to a sensitivity of at least one malignant cell in 10 000 or higher in greater than 90% of patients and were able to generate risk-based data in 79% (112 of 142) of patients who completed the induction and consolidation. Five-year OS was 72% for MRD− and 14% for MRD+ patients (P = .001). These findings reflect a significantly reduced cumulative incidence of relapse rate in the MRD− versus MRD+ group (24% vs 69%, P < .001). Bassan and Hoelzer38 provided an in-depth evaluation of the clinical significance of MRD positivity in Ph− patients. Patel et al39 more recently used PCR amplification of clone-specific Ig or TCR rearrangements in 161 B-lineage Ph− ALL patients. MRD positivity in excess of 1:104 cells was associated with significantly shorter RFS and 9-fold higher relapse rates compared with MRD− patients undergoing conventional chemotherapy or autologous HCT. MRD positivity, however, did not adversely affect outcome in recipients of allogeneic HCT in CR1. Finally, although associated with a low antileukemia effect in adult ALL, Geibel et al40 retrospectively reported RFS in patients who attained MRD negativity and then underwent autologous HCT. These data provide a rationale for introducing MRD-based risk stratification into current investigations. Standardized methodology MRD already is in widespread use in prospective studies across Europe, including the UKALL 14 study. Future US studies should use MRD assessment for the delineation of those at significant risk of treatment failure, in whom intensification of therapy should be evaluated; these efforts await a uniform and validated assay. Only large-scale prospective studies will determine whether MRD+ patients can be cured by the use of up-front allogeneic HCT.
Conclusions
Ph− ALL in adults is a heterogeneous disease affected by many patient- and disease-related factors, including age, immunologic subtype, and clinical, genetic and molecular features. Results of prospective studies using sibling-matched allogeneic HCT demonstrate that this approach is superior to conventional chemotherapy and autologous HCT, with OS rates exceeding 50%, although paradoxically, the greatest benefit is in patients deemed standard risk rather than high risk for poor outcome. However, the ongoing advances in conventional therapy intensity have now yielded results that surpass the control arms in the older and largest chemotherapy versus HCT trials, and therefore ongoing reassessment of the place of HCT needs to be continued. MUD and even UCB grafts can be used successfully in patients who lack a family donor, and RIC rather than MA conditioning appears to provide at least comparable patient outcome results in some studies. Further, this less-intense conditioning facilitates HCT in elderly patients and those who have comorbid conditions. Much of the data for allografting with alternative donors, however, were generated from case series and observational databases and caution must be taken in interpreting them.
Table 5 provides guidance for when to consider performing HCT in CR1 ALL patients age 35-70 years based on patient-, treatment-, disease-, and donor-related factors. There are insufficient studies to provide a definitive algorithm and these recommendations are based on many of the publications and assumptions discussed herein. For example, although MRD positivity indicates that chemotherapy alone likely will result in ultimate failure, it remains unclear whether HCT can salvage such a patient. Emerging data with newer agents such as blinatumumab are beginning to show that MRD+ patients can be rendered MRD− and therefore may be salvageable. Further, although RIC regimens are preferred in the elderly and subjects with comorbid conditions, prospective data are not available to demonstrate that lowering the TRM results in a survival advantage in all patients. Finally, some therapeutic strategies, such as haploidentical donor HCT, cannot be prioritized because these approaches are not in sufficient use. Every effort should be made to enroll eligible patients in clinical trials. When this is not possible, the individual patient factors listed in Table 5 should be used to guide the practitioner's decisions regarding the use of HCT.
Disclosure
Conflict-of-interest disclosure: H.M.L. is a member of the Data and Safety Monitoring Board for Celgene Corporation. A.S.A. has received research funding from Pfizer, Novartis, Genzyme, Sigma-Tau, and Millennium; has consulted for Pfizer Pharmaceutical; has from received honoraria from Sigma-Tau and Pfizer; and is affiliated with the speakers' bureau for Sigma-Tau. Off-label drug use: None disclosed.
Correspondence
Hillard M. Lazarus, MD, FACP, Department of Medicine, University Hospitals Case Medical Center, 11100 Euclid Ave, Cleveland, OH 44106; Phone: 216-844-3629; Fax: 216-844-5979; e-mail: hillard.lazarus@case.edu.