Abstract
Management of acquired hemophilia A is challenging and should be undertaken in close collaboration with a hemophilia center with expertise in the field. Treatment involves controlling and preventing bleeds and using immunosuppression to eradicate the inhibitor. Prompt diagnosis is important to allow early hemostatic treatment and to prevent nonessential invasive procedures. First-line hemostatic treatment should be with a bypassing agent. Recombinant activated factor VII and the activated prothrombin complex concentrate anti-inhibitor coagulant complex (Factor Eight Inhibitor Bypassing Activity, or FEIBA) but equally efficacious but both associated with thrombotic events when used in acquired hemophilia. Immunosuppression should be started as soon as a diagnosis has been confirmed. The combination of steroids and cyclophosphamide may induce more patients into remission than steroids alone. Current data do not suggest that rituximab results in better outcomes. Relapse is common (10%-20%) in the first 6 months after immunosuppression is stopped, and patients need to be followed up regularly to allow early diagnosis and treatment of relapse.
Introduction
Acquired hemophilia A (AHA) is an autoimmune disease caused by Abs that inhibit the function of factor VIII (FVIII). AHA presents with a wide spectrum of symptoms ranging from superficial bruising to life-threatening bleeding and has an incidence of approximately 1.4/million/year.1 It typically affects older people with a median age of approximately 77 years. It is associated with autoimmune diseases, malignancy, and pregnancy, although approximately 50% of cases are idiopathic.1–3 There have been several recent reviews and consensus guidelines that cover the etiology, diagnosis, and presentation of AHA.4–6 This chapter focuses on the treatment of bleeding and immunosuppression to eradicate the inhibitor and to restore normal hemostasis. The evidence base for treatment of AHA has recently been significantly expanded by the publication of European Acquired Haemophilia Registry (EACH2).7–9 An important finding of EACH2 is that diagnostic delay is common and this puts patients at unnecessary risk of severe bleeding.9
Treatment
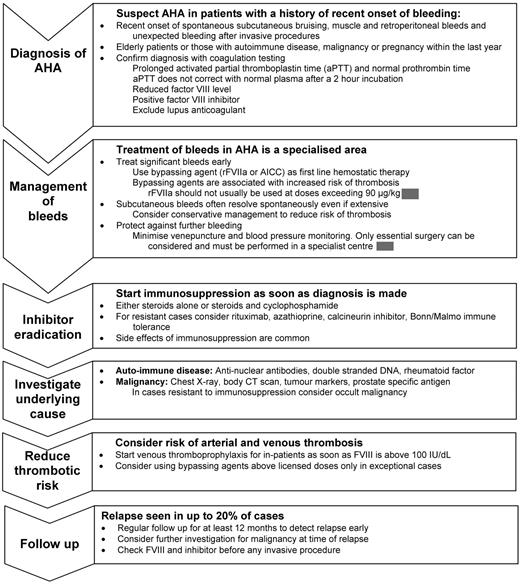
The priorities in the treatment of AHA are to arrest hemorrhage, eradicate the inhibitor, treat underlying disease, and protect against trauma and nonessential invasive procedures (Figure 1 and Table 1).6,10 Patients should be managed in collaboration with a hemophilia center experienced in managing inhibitors even if the initial presentation appears benign, because fatal bleeding can occur at any time until the inhibitor is eradicated.1 Invasive procedures should be avoided and venipuncture and blood pressure monitoring should be kept to a minimum. When patients are managed outside of the hospital, they should be educated to recognize and report symptoms of bleeding early.
Hemostatic management
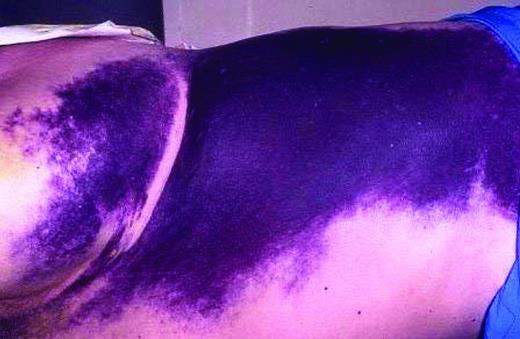
Most data regarding the treatment of bleeds for patients with inhibitors relate to congenital hemophilia and focus on hemarthroses. The autoantibodies that cause AHA have different properties from the alloantibodies seen in congenital hemophilia, and the bleeding pattern differs as well; in particular, hemarthroses are uncommon and soft tissue and mucosal bleeds predominate (Figure 2). This means that data derived from patients with congenital inhibitors cannot necessarily be extrapolated to AHA.
Superficial bleeding in AHA. Superficial bleeding is common in AHA and will often resolve without hemostatic therapy. The risk of thrombotic complications associated with bypassing agents needs to be weighed against the benefit of treatment, especially in patients with additional risk factors for thrombosis. In contrast, other types of bleeds need prompt treatment.
Superficial bleeding in AHA. Superficial bleeding is common in AHA and will often resolve without hemostatic therapy. The risk of thrombotic complications associated with bypassing agents needs to be weighed against the benefit of treatment, especially in patients with additional risk factors for thrombosis. In contrast, other types of bleeds need prompt treatment.
Bleeding in AHA is often very severe and prompt hemostatic control is required to reduce morbidity and mortality. In contrast, EACH2 reported that 144 of 482 patients (30%) did not need hemostatic treatment,7 which confirms the findings of other large cohorts.1 Subcutaneous hemorrhage, even if extensive, can usually be managed without hemostatic therapy, although close observation, usually in the hospital, is required so that intervention can be instituted if necessary (Figure 2). Recent data collected after the introduction of bypassing agents report fatal bleeding in 3%-8% of patients,1,9 in contrast to a study from the 1980s reporting 22% fatality.3 Available hemostatic agents do not have predictable efficacy, so regular clinical review by a physician experienced in the management of inhibitors, supported by appropriate imaging and measurement of hemoglobin level, is important. Early treatment of bleeds is recommended.6 The current options for hemostatic control are the use of bypassing agents, human FVIII (potentially with immunoadsorption), and desmopressin. A period of treatment at reduced dose and frequency after initial bleed control is often needed to prevent recurrence. The regimen by which the intensity of hemostatic treatment is reduced depends on the specific bleed characteristics and requires the input of a clinician experienced in the management of FVIII inhibitors.
Bypassing agents.
At the time of this writing, the available bypassing agents are recombinant activated FVII (rFVIIa; trade name NovoSeven; Novo Nordisk) and the activated prothrombin complex concentrate anti-inhibitor coagulant complex (AICC; trade name Factor Eight Inhibitor Bypassing Activity [FEIBA]; Baxter Healthcare). Almost all published data relate to these agents and results should not be extrapolated to similar agents, because other activated FVII molecules or activated prothrombin complex concentrates may have different properties. A retrospective study of AHA patients treated with rFVIIa combined data from 3 sources with variable inclusion criteria. It reported on 139 patients and 182 bleeds. In the 103 episodes in which rFVIIa was used as first-line therapy, it was effective or partially effective in 95% of cases.11 Similar results were reported in the EACH2 registry, in which 159 prospectively collected bleeds treated first-line with rFVIIa resolved in 92% of cases.7 In the EACH2 registry, 64 bleeds were treated first-line with AICC with 93% resolution.7 In a retrospective study of AICC in 34 patients, moderate bleeds had 100% and severe bleeds 76% hemostatic control at a median of 48 hours.12 When used as second-line therapy, rFVIIa has been reported to have 80% efficacy, and in 57 surgeries, an effective or partially effective response was reported in 86% of cases.11 Mucosal bleeds benefit from concomitant therapy with an antifibrinolytic agent, and reports are emerging that support the safety of this treatment strategy even when combined with AICC.13
Although rFVIIa and AICC have not been compared directly in AHA, a rigorous analysis of data in the EACH2 registry, which controlled for bleed and patient characteristics by propensity score matching, found that the 2 agents had indistinguishable hemostatic efficacy (odds ratio [OR] = 1.0, 95% confidence interval [CI], 0.23-4.44).7 In congenital hemophilia A, studies comparing rFVIIa and AICC for the treatment of hemarthroses also suggest similar hemostatic efficacy.14,15 In EACH2, hemostatic failure was lower with bypassing agents compared with FVIII or desmopressin (OR = 0.15; 95% CI, 0.04-0.53; P=.003).7 Therefore, first-line treatment should be with a bypassing agent, and both rFVIIa and AICC are appropriate options. The choice of bypassing agent will depend on the patient's previous response, convenience of dosing, use of plasma-derived products, and cost. If first-line therapy fails, the alternative bypassing agent may be successful and should be tried at an early stage.
Bypassing agents are associated with thrombosis in AHA. In 139 patients treated with rFVIIa, 12 (8.6%) thrombotic events, mainly arterial, were reported.11 EACH2 reported 11 thrombotic events (7 arterial and 4 venous) in patients treated with a hemostatic agent and 2 in patients not treated with a hemostatic agent. There were 5 of 174 (2.7%) events associated with rFVIIa, 3 of 63 (3.6%) with AICC, 0 of 70 with FVIII and/or desmopressin, and in 3 cases, the hemostatic agent was not reported.7 The incidence of thrombosis in AHA appears to be higher than that reported for congenital hemophilia. Inevitably, there will be more cardiovascular risk factors in patients with AHA because the patient group is older and these patients often have numerous other medical complications. Although a causal relationship between bypassing agents and the reported thrombotic events cannot be established in the studies cited, caution is required and the decision to use a bypassing agent is not straightforward. Treatment of significant bleeding should not be withheld, because the benefit of early control of severe bleeding clearly outweighs the risk of thrombosis. However, minor bleeding (eg, subcutaneous bleeding) should not be treated without careful consideration because these bleeds usually resolve spontaneously. The approach of using increased doses rFVIIa of up to 270 μg/kg has been shown to be safe and efficacious for treating hemarthroses in congenital hemophilia.15,16 This strategy should be considered only in exceptional circumstances in patients with AHA because high-dose rFVIIa has not been shown to be safe in this patient group or efficacious for treating the types of bleeds associated with AHA. The use of combined rFVIIa and AICC should be avoided except in life-threatening situations unresponsive to each agent alone.
Human FVIII.
Human FVIII is often an inadequate hemostatic therapy unless the inhibitor titer is low and, even then, response is unpredictable. FVIII is less effective than rFVIIa or FEIBA for the treatment of bleeds in AHA.7 The dose of FVIII must be sufficient to overcome the inhibitor and provide an adequate hemostatic level. Although formulae have been suggested for calculating the dose, the inaccuracies inherent in the laboratory measurement of inhibitor titers in AHA makes these at best very rough approximations, and regular monitoring of plasma FVIII level and clinical response is required. The use of high-dose human FVIII in combination with immunoadsorption is more likely to result in hemostatic FVIII levels and rapid control of bleeding despite higher anti-FVIII inhibitor titers.17 This treatment strategy may be useful as first-line therapy or if bypassing agents have failed, although it is available in only a very limited number of centers.
Porcine FVIII.
In AHA, the inhibitor titer to porcine FVIII is usually 5%-10% that of the human titer, so porcine FVIII may achieve hemostatic levels in situations in which human FVIII is ineffective. Plasma-derived porcine FVIII has proven efficacy in AHA, but is no longer available.18 A recombinant B-domain–deleted porcine FVIII is under investigation in AHA, and the results are awaited.
Desmopressin.
Some patients with a low-titer inhibitor and baseline FVIII above 5 IU/dL may respond to a desmopressin infusion; however, response is unpredictable and hemostatic efficacy is not as good as that seen with bypassing agents.19 Desmopressin may be useful in treating minor bleeds, but careful laboratory and clinical monitoring of response is required.
Management of surgery.
Invasive procedures are associated with significant risk of severe bleeding because hemostasis cannot be guaranteed. Only procedures that are absolutely unavoidable should be considered. Even then, benefits should be carefully weighed against the risks of treating conservatively until the FVIII level has increased after immunosuppression. Treatment options include the use of bypassing agents, immunoadsorption with FVIII infusion, and, previously, porcine FVIII.
Inhibitor eradication
Patients with AHA should be immunosuppressed to eradicate the inhibitor as soon as the diagnosis has been made, because they remain at risk of fatal bleeding until normal hemostasis has been restored, even if the initial presentation is benign.1,6 There are numerous reports in the literature, but data are often difficult to interpret because variable end points and definitions are used and studies are almost invariably reports of cohorts without controls. The majority of studies are case reports, single-center cohort studies, or retrospective surveys from specialist centers and thus are likely to reflect more severely affected patients and publication bias of good outcomes is inevitable. Approximately 25% of patients have a spontaneous remission, although the associated morbidity is significant. The literature must, therefore, be treated with caution and the conclusions that can be drawn from many studies are limited. The combined information from these types of studies has been reviewed previously.4,5
Options for immunosuppression are steroids alone, steroids with cytotoxics (cyclophosphamide, azathioprine, vincristine, or combination therapy), rituximab, cyclosporin A, plasmapheresis or immunoadsorption, and FVIII immunotolerance. Regimens need to be compared with regard to the proportion of patients achieving complete remission (CR), the time this takes, the relapse rate, and the morbidity associated with the treatment. Recent studies with adequate follow-up have reproducibly reported a relapse rate of 10%-20%, and some patients require long-term immunosuppression to prevent relapse.1,8 The absence of any reported relapses in many published studies suggests that the results need to be interpreted with caution. Meta-analyses have identified older age and underlying malignancy as risk factors for mortality while achieving a CR is protective.2,20
Steroids and cytotoxic agents
The only prospective, randomized study on AHA performed to date enrolled 31 patients. This study is often misinterpreted as providing evidence to support the addition of cyclophosphamide to steroids if a CR has not been achieved by 3 weeks. The study data, however, do not provide evidence that this strategy is superior to any other. Patients were treated initially with prednisolone 1 mg/kg for 3 weeks, after which time 10 patients were in CR. Of the remaining 20 (1 was lost to follow-up), 4 patients were randomized to continuing treatment with prednisolone alone, and this led to CR in 3 (75%). Of the 10 patients randomized to adding cyclophosphamide, 5 (50%) achieved CR and of those in whom cyclophosphamide was substituted for prednisolone, 3 of 6 (50%) achieved CR. There was no difference between the treatment arms.21
A nonrandomized, prospective national consecutive cohort study compared patients treated with steroids versus steroids and cytotoxics. The design of this study made it less prone to selection bias, but the groups were not matched by presenting characteristics. The 34 patients treated with steroids had 76% CR at a median of 49 days (95% CI, 31-62) compared with 78% CR at 39 days (95% CI, 34-57) for the steroids and cytotoxics group. There was no statistically significant difference between the treatment arms and mortality was not different.1 In contrast, a meta-analysis of 20 studies reported that the use of steroids and cyclophosphamide resulted in more patients achieving CR compared with steroids alone.2 A more recent meta-analysis of 32 nonrandomized studies (which included the 20 reports previously used) found that patients receiving combination chemotherapy had a reduced OR of having persistent hemophilia (OR = 0.04; 95% CI, 0.01-0.23) compared with steroid therapy alone (OR = 0.38; 95% CI, 0.14-0.94).20 In these studies, the steroid and steroid/cytotoxic arms were not matched for baseline characteristics.
The most robust analysis available to date comes from the EACH2 registry of 331 patients. Patients treated with prednisone alone were compared with those treated with prednisone and oral cyclophosphamide. The groups were matched for age, sex, inhibitor titer, FVIII level, and underlying etiology by logistical regression and propensity score. The study reported an OR of 3.25 (95% CI, 1.51-6.96; P < .001) in favor of combined therapy despite the prednisone-alone arm receiving a higher dose of steroids.8
Despite the different CR rates after first-line therapy, the final outcome in terms of survival and sustained remission is the same for steroids alone and steroids plus cytotoxics in all large studies.1,2,8 The current data suggest that the combination of steroids and cyclophosphamide is more likely to result in a stable remission than steroids alone, but the final outcome is not better, possibly reflecting increased toxicity of the more intensive regimens. In the EACH2 registry, the combination of steroids and cyclophosphamide was associated with adverse events in 41% compared with 27% with steroids alone. The development of less toxic immunosuppressive regimens in AHA is an important priority.
Regimens involving combination chemotherapy have been reported to have high success rates, but without comparative groups, the results must be treated with caution because the numbers are very small.22 Whichever regimen is used, 3 weeks appears to be too short a time to assess outcome, because the median time to remission has been reproducibly been shown to be approximately 5 weeks.
IVIg
The available evidence strongly suggests that IVIg as a single agent or in combination with steroids and cytotoxics is not useful in inhibitor eradication in AHA. Although a study of 16 patients treated with IVIg reported that 3 subjects with an inhibitor titer ≤ 1 BU/mL achieved an undetectable inhibitor titer and normal FVIII level, one patient also received steroids.23 A larger study compared nonrandomized patients who either did or did not receive IVIg and showed no benefit for IVIg.1 EACH2 reported that of the 33 patients who received IVIg, 45% achieved a stable CR and 55% did not.8
Rituximab
Rituximab is becoming a commonly used treatment for AHA, but case reports, patient cohorts, and reviews of the literature do not support the idea that it is superior to other regimens. Rituximab used alone has been associated with a reduced rate of remission in some studies,8 but other studies report remission rates similar to standard therapy.24 A literature review of 71 patients treated with rituximab and a variety of immunosuppressive agents found a response rate of more than 90%, but the investigators were cautious about interpreting the results because this design would be prone to positive publication bias, so they suggested that rituximab should be used as a second-line agent in combination with steroids.25 Another literature review reported that 42 patients treated with rituximab had similar outcomes to 44 control patients treated with cyclophosphamide and steroids.26 Data from EACH2 support these findings: 30 of 51 (59%) patients treated with a regimen that included rituximab achieved a stable remission. The 12 patients treated with rituximab alone had only a 42% response rate, whereas those treated with rituximab and another agent had a 64% stable CR, similar to 70% stable CR observed for patients treated with steroids and cyclophosphamide.8 Rituximab has not been shown to result in more rapid remission and may be associated with slower remissions. The 51 rituximab-treated patients in the EACH2 registry had a median time to a negative inhibitor of 65 days (interquartile range, 29-144) compared with other regimens, in which the median time was 32-34 days.8 This finding is supported by other studies.24
The current data on rituximab are difficult to interpret. However, there is no published evidence to support the hypothesis that rituximab results in more patients achieving CR or a more rapid response. It is possible that fewer patients relapsed after being treated with rituximab. In EACH2, the adverse event rate for rituximab was 37%, similar to that for treatment with steroids and cyclophosphamide. Some patients resistant to standard first-line regimens respond to second-line rituximab, but there is no evidence to support the use of rituximab in patients with high-titer inhibitors, as has been suggested previously.27
Cyclosporin A
Several cases have been reported in which cyclosporin A has induced CR after failed first-line therapy, and a single-center cohort study also suggests a potential role of cyclosporin A as first-line therapy.28
Immunotolerance
The use of FVIII in conjunction with immunosuppressive agents in AHA has been reported. The rationale is that FVIII stimulates Ab-producing cells into division, making them more susceptible to cytotoxic agents. The lack of adequate controls in these studies means that direct assessment of the role of FVIII cannot be made. A cohort of 35 patients with severe bleeding was treated with a combination of oral cyclophosphamide 1-2 mg/kg daily, prednisolone 1 mg/kg daily, immunoadsorption on day 1-5 weekly, IVIg 0.3 g/kg on days 5-7 weekly, and FVIII 100 IU/kg daily. Rapid control of bleeding was reported with an undetectable inhibitor at a median of 3 days (95% CI, 2-4) and CR in 88% of patients at a median of 14 days (95% CI, 12-17).17 The same team has published updated results on 67 patients with similar outcomes.29 Although no controls are reported and the cost of the FVIII is very high, this treatment appears to rapidly control bleeding and induce CR in those who respond. It may be considered in severely bleeding patients, especially those unresponsive to bypassing agents.
Venous thromboprophylaxis
Remission of AHA may be associated with high FVIII levels and, because patients are likely to have other risk factors for venous thrombosis, they should be treated with appropriate venous thromboprophylaxis once their FVIII level has normalized.
Relapse
Relapse was reported in 20% of 102 patients at a median of 7.5 months (range, 1 week to 14 months) in one study,1 and this finding is supported by EACH2, which showed that 18% of patients treated first-line with steroids, 12% of those treated with steroids and cyclophosphamide, and 1% treated with first-line rituximab relapsed after a median of 4 months.8 Therefore, patients require prolonged follow-up and should be advised to report symptoms of bleeding or bruising early.
Conclusions
Hemostatic management of AHA is a highly specialized area, because some bleeds require aggressive treatment to prevent severe morbidity and death, whereas others are best treated conservatively because of the risk of thrombosis. If treatment is indicated, bypassing should be used and both rFVIIa and AICC are appropriate first-line agents. There is wide consensus that immunosuppression to eradicate the inhibitor should be started as soon as the diagnosis has been made. Available data suggest that a combination of steroids and cyclophosphamide may result in a higher remission rate than steroids alone. Regimens that include rituximab have not been shown to have any advantage over other regimens, and rituximab as a single agent has been associated with variable results, with one study reporting lower response rates than other immunosuppressive regimens.8 Long-term outcome is not affected by the choice of first-line therapy. Until further data become available, it is not possible to make definitive recommendations regarding immunosuppression, and first-line therapy is at the discretion of the clinician based on the clinical circumstances and taking into account the potential side effects of each treatment. If a patient does not respond to first-line steroids, then a cytotoxic agent or rituximab can be added. Similarly, if a patient fails first-line rituximab, then steroids and cytotoxics agents may be successful. Cyclosporin A is a useful second-line option. A regimen based on high-dose FVIII and immunoadsorption can be considered for patients with severe bleeding.
Disclosures
Conflict-of-interest disclosure: The author is on an advisory committee for and has received research funding from Novo Nordisk, Baxter Healthcare, and Inspiration Pharmaceuticals; has received honoraria from Novo Nordisk and Baxter Healthcare; and has been affiliated with the speakers' bureaus for Novo Nordisk and Baxter Healthcare. Off-label drug use: rituximab to treat AHA.
Correspondence
Peter W. Collins, Arthur Bloom Haemophilia Centre, School of Medicine, Cardiff University, Heath Park, Cardiff, Wales, United Kingdom; Phone: 44-29-20-742331; Fax: 44-29-20-744332; e-mail: peter.collins@wales.nhs.uk.