Abstract
Recent advances in multiple myeloma (MM) therapy have led to significantly longer median survival rates and some patients being cured. At the same time, our understanding of MM biology and the molecular mechanisms driving the disease is constantly improving. Next-generation sequencing technologies now allow insights into the genetic aberrations in MM at a genome-wide scale and across different developmental stages in the course of an individual tumor. This improved knowledge about MM biology needs to be rapidly translated and transformed into diagnostic and therapeutic applications to finally achieve cure in a larger proportion of patients. As a part of these translational efforts, novel drugs that inhibit oncogenic proteins overexpressed in defined molecular subgroups of the disease, such as FGFR3 and MMSET in t(4;14) MM, are currently being developed. The potential of targeted next-generation diagnostic tests to rapidly identify clinically relevant molecular subgroups is being evaluated. The technical tools to detect and define tumor subclones may potentially become clinically relevant because intraclonal tumor heterogeneity has become apparent in many cancers. The emergence of different MM subclones under the selective pressure of treatment is important in MM, especially in the context of maintenance therapy and treatment for asymptomatic stages of the disease. Finally, novel diagnostic and therapeutic achievements have to be implemented into innovative clinical trial strategies with smaller trials for molecularly defined high-risk patients and large trials with a long follow-up for the patients most profiting from the current treatment protocols. These combined approaches will hopefully transform the current one-for-all care into a more tailored, individual therapeutic strategy for MM patients.
Introduction
The last decade has seen significant advances in our understanding of the biology of multiple myeloma (MM) and our approaches to its treatment. As a result of these advances, the median survival of patients has increased and we can now achieve a cure in at least some patients. The immunomodulatory drugs and “proteasome-inhibitor” therapies now form the backbone of MM therapy; however, these classes of drugs can clearly be developed further to improve outcomes. Further, despite these advances, there remains a need for therapies with novel modes of action. There is an expanding set of candidate drugs with great potential that are currently under investigation; however, we need to consider how best to evaluate these agents. In traditional drug-development approaches, novel agents are initially evaluated in cell line and animal models and candidates with activity are then tested in end-stage patients. This approach is not without its problems, with a less than optimal correlation between activity in model systems and activity in the patient. In addition, when drugs are evaluated clinically, it is often in cases with relapsed refractory MM. Although these are initially safety studies, a response and outcome signal is used to make the decision to take the agent forward. The molecular mechanisms present in this stage of MM are different from those acting earlier in the disease process and could result in our abandoning active agents that may be active in the early phases of disease. Conversely, persisting with this approach may lead to the development of agents that only work in the relapse setting and have no merit for earlier stages of disease, when the molecular mechanisms are different. Therefore, it makes sense to pause and consider how we are going to continue with drug development in MM.
At the simplest level, there is a need for new in vitro model systems that accurately reflect the response to treatment in the patient. In addition, new clinical trial strategies for the evaluation of novel agents are needed that take into account disease stage and molecular subtype. For example, in the early stage of drug evaluation of a targeted agent in unselected patients, drugs active in a specific molecular subset could be missed. Since the introduction of the immunomodulatory and proteasome-inhibitor drugs, there has been an improvement in overall survival and this needs to be taken into consideration in clinical study design. In both transplantation-eligible and noneligible patients, the overall survival of the control arms in phase 3 studies has improved dramatically. This suggests that larger studies with longer follow-up times will be required in the future to have adequate power to detect meaningful improvements in outcome, slowing down the evaluation process. This chapter considers how we can exploit advances in our understanding of the biology of MM to facilitate the development of new treatments and to direct their use in the clinic. The rapid acceptance of this personalized medicine strategy and its widespread application in the clinical setting is a critical change that can be readily implemented to maintain our current momentum to make MM a curable disease.
Genetic basis of MM
In the classical view of the initiation and progression of MM, an initiating hit is required to immortalize a myeloma-propagating cell (MPC). Such a cell is then destined to acquire additional genetic hits over time, mediated via translocation, loss of heterozygosity, gene amplification, mutation, or epigenetic changes. The acquisition of additional hits further deregulates the behavior of the MPC, leading to the clinically recognized features of MM.1 The basic premise underlying these interactions is that multiple mutations in different pathways deregulate the intrinsic biology of the plasma cell, changing it in ways that generate the features of MM. Many of the genes and pathways mediating this transformation process have now been characterized.
At the cytogenetic level, the MM genome is recognized as being complex.2–4 The study of chromosomal translocations generated by aberrant class-switch recombination shows that several oncogenes, including cyclin D1 (CCND1), CCND3, fibroblast growth factor receptor 3 (FGFR3), the MM SET domain (MMSET; also known as WHSC1), MAF, and MAFB, are placed under the control of the strong enhancers of the heavy chain Ig (IGH) loci, leading to their deregulation.5,6 Deregulation of the G1/S transition is a key early molecular abnormality in MM and the consistent deregulation of a D-group cyclin was first noted as a consequence of studying the t(11;14) and t(6;14) translocations, which deregulate cyclin D1 and cyclin D3, respectively.1 Nontranslocation-based overexpression of a D-group cyclin can also occur, and in the t(14;16) is modulated via MAF, which up-regulates CCND2 by binding directly to its promoter. Patients with the t(4;14), which translocates FGFR3 and MMSET to the IGH enhancers, also overexpress cyclin D2, but in this case the underlying mechanism is uncertain.6 Other IGH translocations are seen in MM and, in contrast to the class-switch recombination–driven events, tend to occur later in the disease process. The gene typically deregulated by such events is MYC, the deregulation of which may lead to a more aggressive disease phase. Translocations outside of the Ig gene loci can also occur and constitute a significant mechanism leading to gene deregulation that has not been explored fully.1 However, it is known that such translocations can range from single to multiple events per patient, but no recurrent events deregulating a single crucial gene have yet been identified.
The frequency and recurrent nature of interstitial loss of copy number and loss of heterozygosity suggests that the minimally deleted regions contain tumor-suppressor (TS) genes that are driver events.3,7,8 Most TS genes require inactivation of both alleles and have either been identified by the study of homozygous deletions or through the integration of mutational analysis with copy number status.9 Examples of relevant TS genes include FAM46C, DIS3, CYLD, baculoviral IAP repeat containing protein 2 (BIRC2; also known as cIAP1), BIRC3, and TNF receptor associated factor 3 (TRAF3).2,3,7,8,10 Deregulation of the G1/S transition by overexpression of a D-group cyclin is a key early molecular abnormality in MM. Conversely, also important are loss of a negative cell-cycle regulator, down-regulation of CDKN2C by loss of chromosome 1p32, and inactivation of CDKN2A by methylation.3,4,11 Inactivation of RB1 also affects this checkpoint and may occur as a result of loss of chromosome 13, which is present in 58% of cases of MM; however, homozygous loss and mutational inactivation of this gene is infrequent.2 Other important regions of loss of heterozygosity include 11q, the site of the BIRC2 and BIRC3 genes; 16q, the site of CYLD; and 14q32, the site of TRAF3.7,8,10 All of these genes are involved in the NF-κB pathway, indicating that up-regulation of NF-κB signaling is important in MM.
The other major set of recurrent genetic abnormalities seen in MM is hyperdiploidy associated with the gain of the odd-numbered chromosomes, including 3, 5, 7, 9, 11, 15, 19, and 21. Interstitial copy number gain associated with increased gene expression or with activating mutations in oncogenes represents another set of “driver” genes that can lead to MM progression. A classic example of this is the amplification of 1q, which potentially harbors more than one relevant oncogene; for example, CDC28 protein kinase 1B (CKS1B), acidic leucine rich nuclear phosphoprotein 32 family member E (ANP32E), BCL9, and PDZK1.3 Interstitial copy number gains consistent with the activation of the NF-κB pathway are also seen, including amplification of NIK (MAP3K14), TACI (TNFRSF13B), and LTBR.8
There are approximately 35 nonsynonymous mutations per case in MM,2,12 which is intermediate between the numbers present in the genetically simpler acute leukemias (8)13 and those present in the more complex epithelial tumors, such as lung cancer (540).14 There are few recurrently mutated genes in MM and, for the most part, these affect known oncogenes. However, a few novel genes have been identified (FAM46C in 13% of cases, DIS3 in 11% of cases) and as the numbers of samples analyzed increases, the incidence of recurrent genes will undoubtedly increase as well. This observation is consistent with a hypothesis in which deregulation of pathways is pathogenically important, rather than the deregulation of a specific gene.
Examples of deregulated pathways include the frequent deregulation of the NF-κB pathway, and strategies targeting this pathway upstream of mutated genes may fail if the presence of activating mutations are not taken into account. The ERK pathway is frequently deregulated (NRAS in 24% of cases, KRAS in 27% of cases, and BRAF in 4% of cases), which suggests the need for a novel treatment strategy targeting this pathway. Deregulation of the PI3K pathway is also important in MM, but in contrast to the RAS pathway, the PI3K pathway is not frequently mutated.2 However, phosphorylated AKT, which is indicative of PI3K activity, is detected in 50% of cases. In addition, DEP domain containing mTOR-interacting protein (DEPTOR), a positive regulator of the pathway, is frequently up-regulated, especially in cases with MAF translocations.15 However, at a molecular level, the cause of increased DEPTOR is currently unknown. The frequency of these events makes MM a good model system in which to evaluate targeted inhibitors of the RAS and PI3K pathways.
Although there has been substantial work on the genetics of MM, little is known about the epigenetic changes leading to disease progression and their impact on treatment resistance. DNA can be modified by methylation of cytosine residues in CpG dinucleotides and, in addition, chromatin structure may be modified via histone modifications such as methylation, acetylation, phosphorylation, and ubiquitination. Both DNA and histone modifications can play a part in modulating gene expression.16 The most important epigenetic change relevant to the pathogenesis of MM is global hypomethylation and gene-specific hypermethylation during the transformation of monoclonal gammopathy of undetermined significance (MGUS) to MM.4 The most pronounced DNA methylation change is seen in the 15% of patients with the t(4;14) translocation, who have increased gene-specific hypermethylation compared with other cytogenetic subgroups. This subgroup overexpresses MMSET, which encodes a histone methyltransferase and transcriptional repressor.17,18 MMSET mediates histone 3 lysine 36 (H3K36) dimethylation, and its deregulation leads to global changes in histone modifications that promote cell survival, cell-cycle progression, and DNA repair.19,20 Other chromatin modifiers are also deregulated in MM, including UTX, a histone demethylase, MLL, KDM6B, and HOXA9, and the full relevance of these modifications needs further validation.2,21
To date, no consistent mutations of DNA-repair genes, apart from possibly ATM, have been identified in MM; however, deletions of 17p occur in 8% of patients at presentation and this frequency increases in the later stages of the disease. The key gene at this site is thought to be TP53, mutations of which are associated with increased genomic instability and impaired clinical outcomes.22,23 Deregulation of miRNAs affecting this pathway have also been described, in particular miR-192 and miR-32. Several studies have produced data suggesting a role for miRNA in both normal plasma cell development and the pathogenesis of MM. In particular, the expression of the miRNA cluster miR-17-92, located at chromosome 13, has been shown to change during the progression of MGUS to MM.24,25 miRNA changes are also known to deregulate several pathways relevant to the pathogenesis of MM, including cell-cycle progression, p53, and MYC.26 The complexity of the genetic deregulation of MM is further enhanced by the recent identification of recurrent mutations in DIS3, FAM46C, and SF3B1 suggesting a potential role for RNA processing.2
It is clear that inherited genetic variation can predispose to the development of MGUS based on a 2-fold increased risk in the families of index cases with MM.27 Molecular epidemiological approaches have been used to gain insights into the earliest genetic factors leading to the development of MM and an increased risk of developing MM is associated with 3 genetic loci located at 2p, 3p, and 7p, identifying the gene pairs DNMT3A/DTNB, ULK4/TRAK1 and DNAH11/CDCA7L, respectively.28 It seems likely that more loci will be identified and that some of these will be subtype specific. In addition, these inherited variants can have an impact on both clinical outcome and the side effect profiles of specific drugs.
Genetics-based risk stratification
It is critical that we should try to utilize these new genetic data for the benefit of patients with MM. Because MM is not a single disease entity, it is not a giant step of logic that treatment should be targeted to the molecular subtype of disease rather than using a “one size fits all” approach. These targeted treatment approaches can be based on predicting subtypes of disease, predicting prognostic groups, or predicting the presence of a molecular lesion that can be targeted with a specific therapy.
Prognostication and molecular subtype prediction
One important way to use molecular data in the clinical setting is to stratify patients for clinical risk status and to use this information to select an appropriate treatment. The presence of a specific cytogenetic lesion cannot simply be interpreted in isolation; several factors need to be taken into account. The clinical and cellular background in which a genetic lesion occurs mediates its prognostic impact. For example, MAF translocations at presentation in MM are associated with a poor prognosis, but when these translocations are present in MGUS, they are not associated with adverse outcomes.29,30 These observations suggest that MAF deregulation alone is not responsible for adverse outcomes and that it probably collaborates with other genetic events (Table 1). This concept underlies the development of the International Staging System (ISS) combined with FISH, which is a significant improvement on the ISS, the previous best prognostic classification in MM.29 Another important observation relevant to predicting clinical outcomes using genetic data is that at disease presentation, genomic events such as the t(4;14) translocation, MAF translocations, gain(1q), and del(17p) can all occur simultaneously.23,29 When each of these abnormalities occurs on its own, the adverse impact on prognosis is less than when multiple lesions occur in the same patient, suggesting that if we are to use these events as prognostic factors, we need to describe and count the presence of all pertinent lesions.29,31 Metaphase cytogenetics only gives information in a subset of cases; in contrast, interphase FISH is universally applicable. Single FISH probes for a range of prognostically important genetic events, including gain(1q), del(17p), and the adverse translocation groups t(4;14), t(14;20), and t(14;16), can be used to define prognosis. However, some patients who are positive for these apparently poor prognostic markers have very good survival.32,33 This issue of poor specificity for the relevant clinical outcome complicates the use of stratified treatment because of the potential risk of either over- or undertreatment with toxic chemotherapy regimens.
Alternate approaches to the definition of high-risk cases have been developed based on gene-expression profiling (GEP). Using these GEP approaches, several signatures have been derived that can be used to risk stratify patients9,34 ; however, these tests are not specific for a given clinical outcome, and high-risk groups defined by this approach also have a range of outcomes. In addition, these types of tests can be difficult to interpret in the laboratory and require consistent quality control, making their widespread uptake in clinical laboratories difficult at this time. GEP can, however, identify most groups of the currently used molecular subgroups of MM.6,34–36 However, at this stage to obtain all of the necessary information for definition of prognostic groups, GEP needs to be combined with FISH analysis. An example of this is the detection of del(17p), one of the most important prognostic factors in MM that cannot be assayed by GEP so its detection remains dependent upon the use of a FISH probe. In the future, however, TP53 mutational analysis may replace the reliance on FISH data. An alternative to GEP is the design of specific real-time quantitative PCR reactions for the key expressed genes underlying the translocation and cyclin D classification and combining these with a limited set of FISH probes that fill in missing data, such as del(17p) and gain(1q), which currently cannot be obtained by these approaches.
Predictive markers and targeted treatment
As an approach to treatment, risk-stratified approaches are suboptimal in MM, and in the near future, targeted treatment based on the presence of a specific molecular lesion predictive for response to that treatment is the likely way forward so that we can achieve personalized cancer care for MM patients. The best illustration of this approach is the treatment of the t(4;14) subtype of MM, which has been associated with poor prognosis.29 At least some recent clinical trial data support frontline treatment with proteasome inhibitors for this subtype37 and, in view of the characteristic oncogene profile, it is the optimum group in which to address the potential role of FGFR3 and MMSET inhibitors. This is a good example of how, by characterizing the biology of an initiating lesion thought to be present in 100% of cells, we can push forward the development of novel targeted treatments. The realization that MMSET is a member of a family of oncogenes with H3K36me2 transferase activity raised the possibility of targeting this activity as a therapy for MM. Therefore, the crystal structure of the MMSET protein is currently being resolved and this information is being used in specific structure-function–based drug design approaches to specifically inhibit the activity of this enzyme. Other translocations such as t(14;16) or t(14;20), leading to MAF or MAFB overexpression, are interesting molecular targets as well. A list of potentially therapeutically relevant molecular subgroups of MM is shown in Table 2. The potential for a targeted treatment approach in MM has significantly increased recently with the availability of data derived from the application of genome-wide sequencing strategies. These strategies have led to the identification of recurrent mutations, such as those in the RAS/MAPK pathway, which can be specifically targeted. However, the best current example of a mutation that can be targeted is BRAF, which mutated in 4% of cases of MM.2 However, for the effective use of such a strategy, there will need to be an accompanying molecular diagnostic strategy able to identify the presence of the V600E mutation. Such variants may only be present in a subclone, which suggests potential difficulties with targeted treatment of these lesions.
Molecular diagnostics: current and future strategies
If we are to pursue a targeted approach, it is important to consider how this can be achieved in the clinical setting. Traditional metaphase cytogenetic analysis is not universally applicable, is labor intensive, and is expensive, making it a poor diagnostic test. In contrast, interphase FISH can give results in nearly 100% of cases. Despite this, it does not have desirable characteristics as a diagnostic test because it is also labor intensive, costly, and requires the application of a wide range of probes to describe sufficient lesions to give reasonable prognostic information. Going forward, changes in technology will allow us to overcome many of these current issues. There are already single nucleotide polymorphism–based mapping arrays that can detect all of the copy number abnormalities in MM, but these would need to be combined with an alternate testing strategy able to detect translocations. It is possible to detect such translocations at reasonable cost using DNA capture techniques for the Ig regions and the application of next -generation sequencing (NGS) technology. Relevant mutations can currently be detected using PCR-based diagnostics or single-stranded conformational polymorphism–type approaches, but the flexibility of NGS can be exploited to detect these variants using specific capture and primer combinations and relevant mutations. We are designing NGS strategies that will detect all of the relevant mutations, copy number abnormalities, and translocations typical of MM, which, when combined with high throughput and batch testing, can deliver all of this information at a fraction of the current costs. It seems likely that this MM-specific strategy is a better way forward than relying on whole-genome or exome sequencing strategies, for which there remain serious issues with cost, throughput, and data handling. The same arguments about the complexity of the data generated from a test apply to expression-based analyses with GEP, in which there are issues with reproducibility. Simpler, signature-specific real-time quantitative PCR tests can be applied more generally and give similar clinical information. It can be argued that what is needed is a uniform MM-specific strategy that can be applied in clinical trials and in routine practice.
The challenges of treatment resistance and targeted therapy
Targeted treatment resistance is a real phenomenon, and several lessons have been learned about the development of treatment resistance in other disease settings that are relevant to the application of this approach in MM. Additional molecular diagnostic strategies that identify resistance mechanisms can help in the application of targeted treatment. Treatment resistance can occur when the target is absent or mutated, and this mandates the development of alternate diagnostic approaches able to detect the presence of the mutated target. An alternate mechanism of resistance is that the target for therapy, such as the chronic myeloid leukemia stem cell, can be quiescent and refractory to treatment; the relevance of this mechanism to MM is unknown.
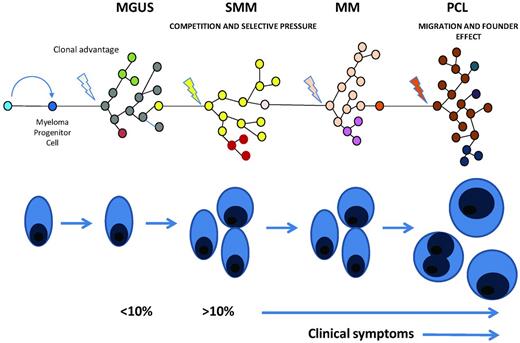
The other broad mechanism that underlies treatment resistance is intraclonal heterogeneity and clonal evolution. Although we and others have shown significant complexity in the genetic basis of MM, the technologies used in these studies reflect the predominant clonal population and fail to take into account the presence of subclonal heterogeneity.2,3 Recent clinical and biological data, however, are consistent with such heterogeneity being a common characteristic of MM. It is likely that, from a Darwinian selection perspective, based on this intraclonal heterogeneity, clonal evolution underlies disease progression and relapse. Based on this knowledge, it is becoming increasingly clear that after disease initiation, the molecular events necessary for MM development are not attained in a linear fashion, but rather via branching, nonlinear pathways typical of those used by Darwin to explain the evolution of the species.38 In this respect, if the MPC evolves according to Darwinian principles, there is the potential for differential responses within the tumor clone and for the selection and expansion of resistant subclones. These observations pose a serious hazard for targeted treatment and also suggest that optimum therapeutic strategies should be targeted at initiating events that develop early in the disease process and not at late events present in a subclone. There are clear examples of how intraclonal heterogeneity of acquired mutations may be relevant to the therapy of MM, and it is interesting that even for a dominantly acting oncogene such as NRAS or KRAS, it is possible to identify variation in the size of the clone carrying the mutation. Therefore, if mutational events are to be targeted, several key pieces of information must be available, such as the presence of a mutation, its driver status, whether the gene is expressed, the activating or inactivating nature of the variant, and the size of the clone carrying it. There is already some evidence for the clinical relevance of this concept with the analysis of gene mapping and massively parallel sequencing data from a patient at multiple time points through their disease, suggesting that treatment can select subclones that may expand and lead to relapse.39 These data suggest that the sequence in which treatments with different mechanisms of action are used may affect clonal selection and thus overall clinical outcomes.
Clonal evolution in myeloma. Illustration of the multistep evolution pathway to plasma cell leukemia. Each step in this process seems to be the result of an acquired growth advantage in a subclone that comes to dominate the clinical picture associated with the transformed plasma cell clone. At each stage the genetic complexity of the disease will increase as each of the prior stages of disease should also be detectable if a test with sufficient sensitivity is available. In plasma cell leukemia because of rapid growth and clonal expansion the prior phases may be present at apparently lower levels.
Clonal evolution in myeloma. Illustration of the multistep evolution pathway to plasma cell leukemia. Each step in this process seems to be the result of an acquired growth advantage in a subclone that comes to dominate the clinical picture associated with the transformed plasma cell clone. At each stage the genetic complexity of the disease will increase as each of the prior stages of disease should also be detectable if a test with sufficient sensitivity is available. In plasma cell leukemia because of rapid growth and clonal expansion the prior phases may be present at apparently lower levels.
Such considerations are relevant to treatment decisions in both the induction and consolidation settings, but are particularly relevant to the use of maintenance therapy, which is showing promise as a treatment strategy in MM. There is a suggestion that thalidomide maintenance may select for resistant clones when used in high-risk disease subsets, and explanations for this can be posed in the context of the model system presented.40 It is clear that induction treatment markedly reduces disease bulk, acting as a classic evolutionary restriction point and resetting intraclonal dynamics. In this context, postinduction therapy could be used to modulate the expansion of more indolent clones favoring long-term survival. In contrast in high-risk biological subsets, such treatment could favor the development of more aggressive clones, potentially reducing postrelapse survival. It is also interesting to postulate, in the context of disease progression, what may happen with the introduction of early treatment in smoldering MM. If there is a dominant clone with indolent behavior that governs access of a more aggressive but minor clone to the MM niche, there may be the potential to enhance disease progression if this indolent clone is eradicated by early treatment. Although exploratory, this Darwinian model of disease progression provides an interesting conceptual framework in which to consider the impact of standard and novel agents used for the treatment of MM and future targeted treatment strategies.
Conclusions
The new insights into MM biology will continue to affect our approach to treating MM. To capitalize on this information, we have to embrace the technologies and approaches that will allow us to apply this knowledge in the clinical setting. As we continue to make progress with favorable-risk MM defined by these technologies, it will be increasingly difficult to carry out small trials over short median follow-up times and expect to gain meaningful clinical information. Therefore, approaches to evaluating drugs in this type of disease will need to change. In contrast, in high-risk disease, we are making less progress and smaller, focused studies may allow us to rapidly evaluate and find new agents for this subgroup. Molecular subgroup–specific trials using targeted agents represent a very important way forward in which we can evaluate the impact of switching off MM relevant signaling pathways using targeted agents. Despite the complexity of this approach, targeting specific molecular lesions represents a very clear way forward for patients with MM, but it demands the development of MM-specific diagnostic platforms and their widespread dissemination through the clinical system in the immediate future.
Acknowledgments
M.F.K. is funded by the Deutsche Forschungsgemeinschaft (DFG KA 3338/1-1).
Disclosures
Conflict-of-interest disclosure: G.J.M. has consulted for Novartis, Celgene, and J&J. M.F.K. declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Gareth J. Morgan, Institute of Cancer Research, Brooks Lawley Building, Sutton, London SW7 3RP, United Kingdom; Phone: 020-8722-4130; e-mail: gareth.morgan@icr.ac.uk.