Abstract
Positron emission tomography/computed tomography (PET/CT) has emerged as the most accurate tool for staging, treatment monitoring, and response evaluation in Hodgkin lymphoma (HL). Accurate staging and restaging are very important for the optimal management of HL, but we are only beginning to understand how to use PET/CT to improve treatment outcome. More precise determination of disease extent may result in more precise pretreatment risk stratification, and is also essential for the minimal and highly individualized radiotherapy volumes of the present era. Several trials are currently investigating the use of PET/CT for early response-adapted therapy, with therapeutic stratification based on interim PET/CT results. Posttreatment PET/CT is a cornerstone of the revised response criteria and enables the selection of advanced-stage patients without the need for consolidation radiotherapy. Once remission is achieved after first-line therapy, PET/CT seems to have little or no role in the routine surveillance of HL patients. PET/CT looks promising for the selection of therapy in relapsed and refractory disease, but its role in this setting is still unclear.
Introduction
Optimal management of Hodgkin lymphoma (HL) demands a careful balance between high treatment efficacy and acceptable acute and late treatment-related toxicity. With modern therapy, overall long-term survival from Hodgkin lymphoma (HL) exceeds 80%,1 but there are serious long-term adverse effects of the treatment, including heart and lung disease and secondary malignancies. HL patients have an excessive mortality directly related to these late treatment effects.2 At 15 years after treatment, the risk of death from HL is overtaken by the risk of death from other causes and, in early-stage HL, treatment-related illness accounts for more deaths than HL itself.3 To reduce the long-term effects of treatment, therapeutic strategies are becoming more tailored to the individual patient's disease profile and other clinical characteristics, with the aim of maintaining and even improving the high cure rates while reducing therapy-related morbidity and mortality.
Positron emission tomography (PET) using 2-[18]fluoro-2-deoxyglucose (FDG) has gained widespread use in most lymphoma subtypes. State-of-the-art FDG-PET is carried out in combined scanners, with FDG-PET and computed tomography (CT) performed in one scanning session, resulting in fusion PET/CT images. The National Comprehensive Cancer Network guidelines recommend the use of PET/CT for primary staging and final response evaluation in patients with HL.4 Interim PET/CT during chemotherapy is still considered to be investigational, and the role of PET/CT in relapsed/refractory disease is also not yet clear.
A more patient-tailored treatment approach demands precise determination of the initial disease extent and also an accurate, and preferably early, assessment of the responsiveness to therapy. Because PET/CT seems to be the most accurate staging tool in HL and provides the most reliable response assessment during and after therapy, the method plays an important role in current efforts to optimize therapy. This chapter explores the role of FDG-PET/CT in the selection of therapy for HL patients, both in the first-line setting and in relapsed disease.
Staging PET/CT and selection of first-line therapy
Decisions on treatment strategy for HL patients rely on determination of histology, on accurate staging of the disease, and on identification of risk factors for early-stage disease or the individual parameters of the International Prognostic Score (IPS) for advanced-stage disease.5 Clinical stage is by far the most important determinant for the choice of up-front treatment strategy. The first reports on FDG-PET for lymphoma imaging were published 25 years ago.6 Studies of HL patients showed a very high sensitivity of FDG-PET for nodal staging, especially for the detection of peripheral and thoracic lymph nodes. When performed as PET/CT, the increased sensitivity does not come at the expense of a decreased specificity.7 PET/CT also detects extranodal disease more sensitively than conventional methods, both in the BM and in other organs and seems to be at least as sensitive as blind BM biopsy (BMB).7,8 A recent study of 454 HL patients with staging BMB and PET/CT showed no value of routine BMB in the era of PET/CT staging.9
PET/CT has a consistent, large influence on the staging in classical HL, with upstaging of approximately 15%-25% of patients and downstaging in only a small minority of patients. This leads to a shift to a more advanced treatment group in approximately 10%-15% of patients.7,10,11 A single study showed a similar pattern in nodular lymphocyte–predominant HL, in which staging FDG-PET resulted in changes of stage in 9 of 31 patients (7 upstagings and 2 downstagings).12 The tendency toward upward stage migration is important, because HL is a disease in which the early and advanced stages are treated very differently. Early-stage HL patients have an excellent prognosis but are prone to serious treatment-related late morbidity and mortality. With this in mind, the use of FDG-PET/CT for staging of HL should be accompanied by steps to reduce the intensity of therapy, or the net effect of the enhanced staging accuracy will be an increased overall treatment burden.
The role of baseline PET/CT for modern early-stage HL radiotherapy planning
In modern radiotherapy for HL, extended fields developed for single-modality treatment have now been increasingly replaced by treatment encompassing the initially macroscopically involved tissue volumes in early-stage disease and bulky masses and/or residual masses after chemotherapy in advanced disease.13 These changes have led to dramatic reductions in the volume of normal tissue being irradiated and similar reductions in the risk of serious late effects of radiotherapy.14,15 However, such modern therapy demands a higher accuracy of the imaging procedures used for treatment planning. Because PET/CT is more accurate for staging of HL, it is by implication also more precise in defining the initially involved regions or nodes that are intended to be irradiated in patients with early-stage disease. In radiotherapy for early-stage HL, the initial lymphoma volume seen on the staging PET/CT scan must be contoured on a planning scan done after chemotherapy. Image fusion may be used to allow prechemotherapy images to be combined with the postchemotherapy images, thus aiding in the accurate delineation of the initially involved nodes. Relatively limited clinical data are available on the role of PET/CT in target definition for the planning of radiotherapy for HL. However, in the setting of modern conformal radiotherapy techniques such as involved-field and involved-node radiotherapy, the definition of the involved nodes and thus the radiotherapy volumes is significantly different with PET/CT compared with CT alone, both in classical and nodular lymphocyte–predominant HL.12,16,17
Early treatment monitoring with FDG-PET
Clinical stage and prognostic factors are used to determine the initial treatment strategy for HL. However, the tumor response to induction treatment is strongly prognostic. A reliable and early prediction of response to therapy may identify good-risk patients who will be cured with conventional therapy—or even with less-intensive and less-toxic regimens—and poor-risk patients for whom an early switch to alternative, more aggressive treatment strategies could improve the chance of remission and cure. This concept, called risk-adapted therapy, is widely recognized as one potential way to achieve higher cure rates without increasing (and perhaps even decreasing) the risk of treatment-related morbidity and mortality.18
Conventional methods for treatment response monitoring are based on morphological criteria, and a reduction in tumor size on CT is the most important determinant.19 However, size reduction is not necessarily an accurate predictor of outcome. In HL, the malignant cells make up only a small fraction of the tumor volume, which is dominated by reactive infiltrating cells not directly affected by antineoplastic therapy.20 Even more importantly, tumor shrinkage takes time and depends on several factors in the host, so the rate of structural regression cannot form the basis for therapy response assessment until rather late during treatment, at which point a treatment modification might be less useful.
As opposed to the morphological changes of the lymphoma occurring later during therapy, functional imaging with FDG-PET enables early evaluation of the metabolic changes that take place very early during the treatment induction. Several studies of FDG-PET after 1-3 cycles of chemotherapy21–25 have shown that these early metabolic changes are highly predictive of final treatment response and progression-free survival (PFS). The most evidence is available for FDG-PET after 2 cycles of chemotherapy; however, there are data to suggest that the prognostic accuracy is very high already after only one cycle of chemotherapy, and that the negative predictive value (NPV) may be higher (Hutchings et al, manuscript in preparation, and Kostakoglu et al26 ).
A retrospective analysis of 88 patients scanned after 2 or 3 cycles of ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine)–like chemotherapy for HL showed a 5-year PFS of 39% in the PET+ group compared with 92% in the PET− group.21 These results were later confirmed in several prospective studies,22–24 showing excellent outcomes for early PET− patients (approximately 95% long-term PFS) and rather poor outcomes for early PET+ patients. In patients with advanced disease, the high prognostic value of early FDG-PET overshadows the role of the IPS.22,25 The prognostic value of PET/CT in advanced HL was recently validated by Gallamini et al, who showed 3-year failure-free survival of 28% and 95% for early PET+ and early PET− patients, respectively (Gallamini et al, manuscript submitted). In this international validation study, the interobserver agreement was very high between 6 independent PET/CT reviewers using the Deauville criteria for interim PET, which have become widely recognized.27 Apart from giving reproducible results, the Deauville criteria are very simple to use, so their use in most of the recently opened PET response-adapted trials will hopefully enhance comparability between clinical trials and enable a better translation of clinical trial results into clinical practice outside of trials.
The positive predictive value of early FDG-PET seems to be lower in patients treated with the more dose-intensive BEACOPP escalated (BEACOPPesc) regimen than in patients treated with ABVD.28 In addition, the positive predictive value is lower in patients with early-stage HL, probably due to both the inherent better prognosis for this patient group and due to the subsequent radiotherapy that may in many early-stage patients overcome an insufficient chemotherapy response.22,23
PET-response adapted HL therapy: early and advanced stage
There is still no evidence that HL patients benefit from having treatment adapted according to the results of early PET/CT. More than 90% of early-stage HL patients are cured with standard therapy. However, these patients still have a dramatically reduced life expectancy due to treatment-related illnesses including second cancers and cardiopulmonary disease. In fact, more early-stage HL patient die from late effects of therapy than from the disease itself.29 This suggests that a substantial number of early-stage HL patients are subject to some amount of overtreatment, and this is an argument for using early PET/CT to identify good-risk, early-stage patients eligible for less-intensive treatment. Several trials have investigated such PET-response adapted therapy in early-stage HL (Table 1). The United Kingdom National Cancer Research Institute (NCRI) Lymphoma Group RAPID trial for early-stage patients and the German Hodgkin Study Group (GHSG) HD16 protocol investigated the noninferiority of reducing treatment intensity by omitting radiotherapy to interim PET− early-stage patients. The experimental arms of the European Organisation for the Research and Treatment of Cancer, Groupe des Etudes des Lymphomes de l'Adulte, and Fondazione Italiana Linfomi (EORTC/GELA/IIL)1 H10 protocol also omitted radiotherapy to PET− patients while escalating to BEACOPPesc followed by radiotherapy in PET+ patients. The latter trial therefore tests the noninferiority of a less-toxic treatment to good-risk patients while at the same time attempting treatment intensification for patients regarded as having a high risk of failure based on a positive interim PET/CT. The German HD16 trial is still recruiting patients, and results from the United Kingdom RAPID trial and the EORTC/GELA/FIL H10 trial have not yet been published.18 The experimental arms for early PET− patients in the H10 trial was closed after a futility analysis of interim data found that it was unlikely that noninferiority of the chemotherapy-only treatment could be demonstrated compared with the combined-modality standard arms.
Studies of early PET response-adapted HL therapy
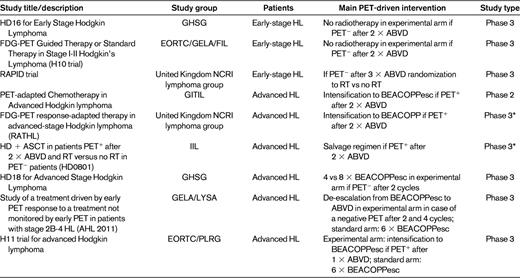
DLBCL indicates diffuse large B-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; and R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
*No randomization regarding PET response-adapted therapy.
Around 70% of advanced-stage HL patients are cured with 6-8 cycles of ABVD with or without consolidation radiotherapy, which is first-line therapy in most centers. BEACOPPesc cures 85%-90% of patients if given upfront, but serious concerns regarding acute toxicity and second myeloid neoplasias are the reason that many centers in Europe and North America are very reluctant to use this regimen as standard therapy.30 Several trials investigating PET response-adapted therapy for advanced-stage HL patients are ongoing (Table 1). Several nonrandomized trials are studying early treatment intensification with BEACOPPesc (the Italian Gruppo Italiano Therapie Innovative nei Linfomi [GITIL] and the United Kingdom-Nordic Response-adapted Therapy in Hodgkin Lymphoma [RATHL] trial2 ) or even ASCT (the Italian FIL trial) in patients who are still PET+ after 2 cycles of ABVD. The randomized German GHSG HD18 trial tests abbreviation of BEACOPPesc therapy based on PET results after 2 therapy cycles. The French AHL 2011 trial is also a BEACOPP-based randomized trial with treatment modifications based on PET after both 2 and 4 cycles. The recently opened EORTC/Polish Lymphoma Research Group (PLRG) H11 trial compares BEACOPPesc (standard arm) against an experimental arm in which PET/CT after one cycle of ABVD determines whether patients continue with ABVD or BEACOPPesc. As with the trials in early-stage disease, none of the PET response-adapted trials in advanced HL have reached mature results at the time of this writing.
Postchemotherapy PET/CT for selection of advanced-stage patients for consolidation radiotherapy
In advanced disease, radiotherapy is used less frequently and usually only for residual disease. In this situation, PET/CT may help in discriminating between a residual mass with viable lymphoma cells and a residual mass consisting only of fibrotic tissue. However, because PET/CT cannot detect microscopic disease, it has not been entirely clear whether a PET− residual mass requires radiotherapy. The mature results of the German HD15 trial shed light on this for patients treated with BEACOPPesc regimens. In that study, consolidation radiotherapy was given only to patients with a PET+ residual mass of more than 2.5 cm. The remaining majority of patients who did not receive radiotherapy had a relapse-free survival of 94% after one year, indicating that radiotherapy can be safely omitted in advanced-stage HL patients who are PET− at the end of BEACOPPesc. The situation is a little less clear for ABVD-treated patients. A retrospective analysis from the British Columbia Cancer Agency was presented at the Annual Meeting of the American Society of Clinical Oncology in 2011. This retrospective study reported a 5-year experience in which patients with residual masses of > 2 cm after chemotherapy underwent PET/CT (n = 163). Only patients with a positive posttreatment PET/CT received radiotherapy. Of the patients with a negative PET/CT (n = 130, 80%), the 3-year PFS was 89% with a median follow-up of 34 months; PET+ patients had a 3-year PFS of 55% despite receiving radiotherapy.31 These results strongly support the omission of radiotherapy in advanced-stage HL patients who receive a PET− remission after 6 cycles of chemotherapy.
PET/CT for final response evaluation
An extensive number of studies have shown that FDG-PET performed after treatment is highly predictive of PFS and OS (overall survival) in HL patients with and without residual masses on CT.32,33 Based on these findings, the International Harmonization Project developed recommendations for response criteria for aggressive malignant lymphomas that incorporate PET/CT into the definitions of end-of-treatment response in FDG-avid lymphomas, including HL.34 Subsequent retrospective analyses confirm the superiority of the new response criteria in HL compared with the previous criteria based on morphological imaging alone.35 The new recommendations for response criteria are not as yet supported by substantial amounts of clinical data. Long-term follow-up of lymphoma patients evaluated by these criteria should be widely reported and is awaited with great interest. It should be kept in mind that a negative PET/CT does not rule out the presence of microscopic disease, just as a positive PET/CT does not establish treatment failure without verification by biopsy.
PET/CT during follow-up
Tumor burden is a prognostic factor at the time of HL relapse, but there is no evidence that relapsing patients with minimal, asymptomatic disease do better after salvage therapy than patients with low tumor burden and discrete symptoms; routine surveillance imaging should also be viewed in this perspective. PET/CT seems to be the most sensitive method to detect an asymptomatic HL relapse. However, due to a high number of false-positive scans, the positive predictive value of PET/CT is low during routine follow-up, as is the cost-effectiveness. In the largest study to date, PET/CT detected some relapses earlier than CT would have, but it took 50-100 PET/CT scans to speed up the detection of one relapse.36 More recent studies also show a high number of false-positive results, high costs, and limited added value when PET/CT is used routinely in the follow-up setting.37,38 Based on the available literature, PET/CT cannot be recommended for routine follow-up of HL patients who have achieved remission after first-line therapy. Conversely, due to the high negative predictive value, PET/CT is the method of choice to investigate a clinically suspected relapse.
PET/CT before high-dose salvage therapy in relapsed HL
Duration of remission before relapse, and the response to induction therapy are important prognostic factors that predict a good outcome after high-dose chemotherapy with autologous stem cell support (HD + ASCT). Several studies have shown that PET/CT performed after induction therapy and before HD + ASCT can predict which HL patients will achieve long-term remission after the salvage regimen.39–41 These studies all report a poor long-term PFS (after 2-5 years) in patients who are PET+ after induction chemotherapy (31%-41%) compared with a PFS of 73%-82% in the patients who reach a PET− remission before HD + ASCT. However, these studies also report a higher false-positive rate than with PET/CT performed early during first-line therapy. The role of PET/CT in this setting is still unclear, but the available evidence calls for clinical trials to improve the outcomes for patients who are still PET+ after induction salvage chemotherapy.
PET/CT before and after ASCT for relapsed HL
Little is known about the value of PET/CT in patients who relapse after or are ineligible for HD + ASCT. There are data to suggest that the remission status determined by PET/CT before ASCT with reduced-intensity conditioning is highly predictive of outcome.42 Two studies indicate that after ASCT, PET/CT may have a role in guiding the use of donor lymphocyte infusions.43,44
Conclusions
PET/CT has become the most important imaging modality in the management of HL. Its use is based on much evidence and it clearly enhances the quality and accuracy of staging, response assessment, and treatment evaluation. Improved staging accuracy is in itself not very likely to translate into any detectable improvements in patient outcome; this is the case for PET/CT just as it was for CT. Although PET/CT seems to surpass any other existing tools in terms of diagnostic and prognostic properties, its clinical value to the patients depends on the way clinicians use it. There is a general agreement that it is desirable to have access to the most accurate determination of disease extent at the time of diagnosis and access to the most prognostic assessment of final treatment response. For those reasons, PET/CT has been accepted as standard of care at staging and final response assessment of HL, and therefore has been incorporated into the current guidelines.
The situation is less clear during therapy. PET/CT allows for a better prediction of final treatment response and long-term outcome than CT. In the absence of clear evidence that interim PET/CT leads to improved survival, some investigators argue against the use of PET/CT during therapy. This is based on a concern that the results of PET/CT may be wrongly used to intensify therapy when patients are already at risk of overtreatment. Clearly, PET/CT should not lead to clinical consequences that are not evidence based, but this should probably not discourage us from using the most accurate treatment monitoring. If this were the case, then we should also be discouraged from using PET/CT at staging (which may worsen the problem of overtreatment if not used wisely) and even from using CT-based treatment monitoring (which has certainly never been shown to improve outcomes). Although PET/CT is an excellent tool for HL staging and treatment monitoring, we are only beginning to understand how best to use this tool. To keep improving this understanding, we should continue to offer our patients treatment within clinical trials investigating risk- and response-adapted HL therapy. In clinical use outside of the context of clinical trials, it is important to avoid the inappropriate use of PET/CT, and particularly to avoid using PET/CT results to guide therapeutic decisions if they are not supported by evidence from clinical trials.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Takeda/ Millennium. Off-label drug use: None disclosed.
Correspondence
Martin Hutchings, MD, PhD, Department of Haematology, The Finsen Centre, Rigshospitalet, Copenhagen University Hospital, 9 Blegdamsvej, DK-2100 Copenhagen Ø, Denmark; Phone: 45-35459696; Fax: +45-35454295; e-mail: martin.hutchings@gmail.com.
