Abstract
Acute myeloid leukemia (AML) is the most common acute leukemia diagnosed in adults, and the majority of patients with AML die from relapsed disease. Although many studies over the past 4 decades have identified disease alleles in AML, recent genome-wide and candidate gene studies have identified additional recurrent somatic mutations in AML patients with biologic, clinical, and therapeutic importance. Herein we review our current understanding of the molecular pathogenesis of AML and discuss how mutational profiling can be used to refine prognostication in AML and to inform therapeutic approaches. We also review the current challenges in translating genomic studies to the clinical setting, which remains a significant challenge and an urgent priority.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia diagnosed in adult patients. Although most patients achieve complete remission with induction chemotherapy, the majority of patients relapse after achieving clinical remission. The use of consolidation therapy with high-dose cytarabine or stem cell transplantation improves outcomes in adult AML patients; however, despite the use of intensive consolidation strategies, outcomes for AML patients remain poor. Novel therapies are needed for patients with AML based on novel insights into AML pathogenesis. In addition, there is significant heterogeneity in outcome in AML; some patients are cured with the used of induction and consolidation chemotherapy alone, whereas others require allogeneic stem cell transplantation for curative intent or develop relapsed, refractory disease that is not amenable to current pharmacologic or immunologic approaches. Therefore, there is a pressing need to identify biomarkers that accurately predict outcome in AML so that patients are matched to the most appropriate therapies based on prognostic risk and therapeutic response.
In the current clinical setting, a relatively small subset of genetic abnormalities are used to predict outcome and to direct therapy in AML. These include CBF translocations and translocations associated with acute promyelocytic leukemia, which predict for favorable outcome with induction/consolidation and for sensitivity to all-trans retinoic acid and arsenic trioxide, respectively. More recently, based on work by Schlenck et al and others in the field, it has been shown that mutational analysis of FLT3 in combination with NPM1 or CEBPA mutations can be used to predict outcome in normal karyotype AML and to identify patients who will benefit from allogeneic stem cell transplantation.1 However, a large number of AML patients lack any of these abnormalities and there remains significant heterogeneity in clinical outcome within currently classified prognostic groups. These observations suggest there are additional biomarkers that can predict outcome in AML. Recent genetic studies have identified an increasing number of recurrent somatic mutations in AML patients, including mutations in TET2,2,3 ASXL1,4,5 IDH16 and IDH2,7,8 DNMT3A,9–11 and PHF6.12 In addition, several of these newly identified genetic abnormalities have been shown to have prognostic importance in AML. Herein we review the prognostic relevance of novel AML disease alleles and discuss how genetic data can be used to inform outcome and therapy in AML.
Identification of novel disease alleles in AML patients
TET2 mutations
In 2009, 2 groups of investigators mapped microdeletions and copy neutral loss of heterozygosity on chromosome 4q24 to identify recurrent somatic mutations in TET2 patients with myelodysplastic syndrome (MDS) and with myeloproliferative neoplasms.2,13 Subsequent studies of large cohorts of patients with a spectrum of myeloid malignancies identified TET2 mutations in a spectrum of myeloid malignancies, including in 10% of AML patients.3 TET2 is a member of the TET family of proteins. Recent biochemical studies have identified a novel epigenetic function for this class of enzymes, specifically the Fe(II) and alpha-ketoglutarate dependent conversion of 5-methylcytosine to 5-hydroxymethylcytosine.14 5-hydroxymethylcytosine serves as an intermediate step to DNA demethylation, consistent with the TET family enzymes functioning to remove DNA methylation and with increased promoter methylation in mutant AML patients.15 In addition, recent functional studies using murine models demonstrated that loss of Tet2 in the hematopoietic compartment results in increased self-renewal and in impaired hematopoietic differentiation in vivo.16–19
ASXL1 mutations
Analysis of array-comparative genomic hybridization–based copy number data for somatic alterations in epigenetic modifiers led to the identification of somatic deletions and mutations in ASXL1 in MDS patients.4 Similar to TET2, ASXL1 mutations are found in a spectrum of myeloid malignancies, including myeloproliferative neoplasms, MDS, AML, and, most commonly, chronic myelomonocytic leukemia.4,20 ASXL1 mutations are relatively uncommon in younger patients with AML (occurring in 3%-5% of patients),21 but the prevalence of ASXL1 mutations is increased in elderly patients with AML (occurring in approximately 16% of patients).22 Given that the majority of ASXL1 mutations are somatic nonsense or frameshift mutations, it has been postulated that ASXL1 mutations result in loss of ASXL1 function; however, the precise mechanism of transformation remains elusive.23
IDH1 and IDH2 mutations
IDH1 mutations were first identified in exome-sequencing studies of patients with malignant glioma,24,25 and subsequent whole-genome sequencing efforts identified recurrent IDH1 mutations in AML.6 Later candidate gene-sequencing studies identified IDH2 mutations in AML,7,8 including leukemia-specific recurrent mutations at residue R140. Mutations in IDH1 and IDH2 occur in approximately 15%-30% of patients with AML, with an increased frequency of IDH mutations in older AML patients.
IDH1 and IDH2 are enzymes critical in the Krebs cycle that normally convert isocitrate to alpha-ketoglutarate in a NADP+-dependent manner. The presence of recurrent somatic mutations at highly conserved arginine residues in the active site of IDH1 and IDH2 led investigators to search for novel, neomorphic functions that contribute to malignant transformation. Metabolomic and enzyme-profiling studies demonstrated that mutant IDH proteins acquire a neomorphic enzymatic activity that converts alpha-ketoglutarate to 2 hydroxyglutarate (2-HG).26 2-HG can be detected in vast excess in the serum and BM of AML patients with IDH1/2 mutations,8,27 suggesting that it may serve as a biomarker for this genetically defined subset of AML patients and as a measure of residual disease after AML therapy. However, the pleiotropic roles by which the IDH mutant–mediated production of 2-HG contributes to leukemia pathogenesis has not been fully delineated. Based on mutual exclusivity between IDH1/2 and TET2 mutations in AML, it was demonstrated that IDH mutant–mediated 2-HG production can inhibit the function of the alpha-ketoglutarate–dependent TET enzymes and lead to hypermethylation in AML.15 Expression of IDH mutants in hematopoietic cells causes increased expansion of hematopoietic stem cells and impaired hematopoietic differentiation, similar to what is seen with loss of Tet228 and consistent with a convergent mechanism of leukemic transformation.
PHF6 mutations
Mutations in PHF6 were first identified in T-cell acute lymphoblastic leukemia using next-generation sequencing of the X chromosome.29 The majority of mutations are nonsense or frameshift alleles, again consistent with a loss-of-function role for PHF6 in leukemogenesis. Subsequent candidate gene sequencing of PHF6 in AML revealed that approximately 3% of patients harbored PHF6 mutations.12 PHF6 is a plant homeodomain finger–containing protein, an important highly conserved domain important in recognizing protein-DNA interactions and histone modifications. In T-cell acute lymphoblastic leukemia, loss of PHF6 is associated with expression of the oncogenes TLX and TLX3, and the genetic data are consistent with PHF6 functioning as a X-linked tumor suppressor. The role of PHF6 mutations in AML pathogenesis has yet to be elucidated.
DNMT3A mutations
The first report of somatic mutations in DNMT3A came from studies using targeted next-generation sequencing, which led to the identification of recurrent mutations at the highly conserved R882 residue.9 Later that year, 2 studies using whole-genome and whole-exome sequencing identified DNMT3A mutations throughout the entire open reading frame at a high frequency in AML.10,11 In these 2 studies and in later reports, DNMT3A mutations have been found in more than 20% of adult AML patients, making it the second most common somatic mutation, after FLT3 mutations, in de novo AML identified to date.
DNMT3A functions as a de novo methyltransferase that methylates cytosines in CpG dinucleotides. However, the specific role of DNMT3A mutations in altering epigenetic patterning and in AML pathogenesis has not been delineated conclusively. The genetic data are in part consistent with a tumor-suppressor role for DNMT3A in AML, because many patients present with frameshift or nonsense mutations. However, approximately 50% of AML patients present with mutations at codon R882 while retaining the second, wild-type DNMT3A allele, which is consistent with potential acquisition of a neomorphic function for DNMT3A point mutations. Initial studies of DNA methylation using array-based platforms have not identified significant alterations in global methylation levels in DNMT3A mutant AML patients,10 suggesting that the effects of DNMT3A mutations on DNA methylation are likely site and context specific. More recent studies of Dnmt3a-deficient mice have revealed that loss of Dnmt3A in hematopoietic stem cells results in impaired cell differentiation, increased self-renewal with serial transplantation, and expansion of the hematopoietic stem cell pool.30
Prognostic relevance of newly identified genes
Although a series of elegant studies over the past decade have identified a large set of mutations and overexpressed genes with prognostic relevance in AML,31 in the clinical setting, most AML centers use cytogenetic abnormalities and a relatively small set of gene-based tests to assign risk in AML and to determine postremission therapy. The relative paucity of clinically used biomarkers is due to several factors. First, most biomarker studies focus on a specific genetic lesion and its prognostic relevance without considering the complete set of known mutations in parallel to determine, which mutations predict outcome independently in AML. Second, most studies consider each mutant allele as a distinct variable without considering complex genotypes in which the presence/absence of multiple disease alleles has different effects on outcome than individual mutations by themselves. Third, many studies have focused on mutational “hotspots” or have used less sensitive techniques to identify loss-of-function mutations in large tumor suppressors. Finally and most importantly, until recently, most studies of AML were relatively small in size and/or were not derived from clinical trial cohorts in which the effects of treatment on outcome can be controlled and investigated.
Given the increasing number of genetic abnormalities that have been identified in AML patients, it has become important to determine the prognostic relevance of all known recurrent genetic abnormalities in a uniformly treated AML patient cohort. The largest such study to date used high-throughput resequencing21 of TET2, ASXL1, DNMT3A, PHF6, WT1, TP53, RUNX1, EZH2 PTEN, FLT3, NPM1, CEBPA, HRAS, KRAS, NRAS, KIT, IDH1, and IDH2 in 502 patients from the Eastern Cooperative Oncology Group (ECOG) E1900 trial.32 This trial evaluated the use of anthracycline dose intensification during induction therapy. A total of 657 patients between the ages of 17 and 60 years with de novo AML were randomized to receive standard induction with 45 mg/m2 of daunorubicin plus cytarabine or to receive dose-intensive induction with 90 mg/m2 of daunorubicin plus cytarabine. This large, homogeneously treated patient cohort allowed for extensive mutational profiling from diagnostic samples and correlation with outcome, including disease-free and overall survival and response to induction therapy.
Integrated genetic analysis revealed 3 recently identified genes with prognostic importance in the total cohort of AML patients. Specifically, IDH2 R140 mutations, but not IDH2 R172 mutations or IDH1 mutations, were associated with improved overall survival in the entire E1900 cohort. These data suggest that there are important qualitative and/or quantitative differences in the various IDH1/2 mutations in AML, and that these allele-specific differences in biology have relevance to outcome in AML. In addition, mutations in ASXL1 and PHF6 were associated with adverse overall survival in AML, suggesting that these relatively rare AML disease alleles mark a subset of patients with adverse outcome and that biologic studies are needed to determine how these mutations contribute to leukemogenesis.
Although the identification of single gene mutations with impact on outcome is of clinical and biologic importance, the main goal of mutational studies should be to inform and improve prognostic algorithms in AML. Given the established importance of the 3 broad cytogenetic risk categories in AML, it is therefore important to determine whether mutational status for specific mutations or for combinations of mutations affect outcome in different cytogenetic risk categories. However, to date, most studies have failed to identify robust predictors that modify outcome in patients with favorable or unfavorable cytogenetic risk, suggesting that chromosomal lesions remain the best predictor of outcome for the 40% of AML patients with favorable or unfavorable karyotypic risk.
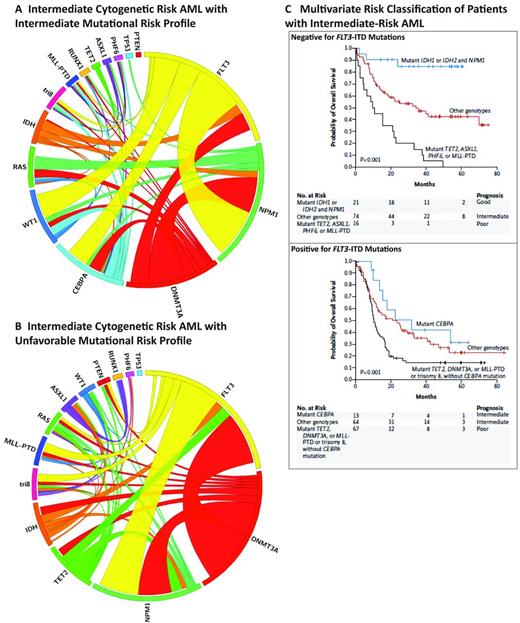
In contrast, mutational studies have been able to increasingly refine prognosis in patients with intermediate-risk and/or normal karyotype AML. Previous studies have suggested that mutational analysis of CEBPA, NPM1, and FLT3-ITD can be used to risk-stratify intermediate-risk AML patients.1 However, more extensive mutational analysis better discriminates intermediate-risk AML patients into robust, clinically relevant risk groups (Figure 1).21 In the subset of patients with FLT3-ITD–negative intermediate-risk AML, there are 3 distinct risk groups that are based on mutational status and have vastly different outcomes. FLT3-ITD–negative, NPM1/IDH mutant patients have outcomes that are better than patients with inv(16)- or t(8:21)-positive AML, suggesting that this represents a favorable-risk AML subset defined by a specific mutational genotype. In contrast, FLT3-ITD–negative NPM1 mutant patients without concurrent IDH mutations have a much less favorable outcome. Most importantly, the presence of poor-risk mutations, specifically TET2, ASXL1, PHF6, and/or MLL-PTD is associated with very adverse overall survival for FLT3-ITD wild-type, intermediate-risk patients. These data suggest that NPM1 mutational status alone does not define a favorable subset of intermediate-risk AML, and that the presence or absence of additional disease alleles defines relapse risk in FLT3-ITD wild-type, intermediate-risk AML.
Mutational complexity and prognosis in intermediate-risk AML. (A-B) Circos plots of AML patients with cytogenetically defined intermediate-risk disease with intermediate mutational risk (A) or unfavorable mutational risk (B). (C) Survival curves for FLT3-ITD–positive and negative intermediate-risk disease (used with permission from Patel et al21 ).
Mutational complexity and prognosis in intermediate-risk AML. (A-B) Circos plots of AML patients with cytogenetically defined intermediate-risk disease with intermediate mutational risk (A) or unfavorable mutational risk (B). (C) Survival curves for FLT3-ITD–positive and negative intermediate-risk disease (used with permission from Patel et al21 ).
In addition, mutational studies allow for improved prognostication in intermediate-risk patients who are FLT3-ITD–positive. Among FLT3-ITD mutant patients, concurrent mutations in TET2, DNMT3A, MLL-PTD, or trisomy (8) were associated with very poor outcome. This is a relatively large subset of patients and approximately 47% of the total intermediate-risk FLT3-ITD patient subset fall into this very-high-risk subset. Therefore, the category of intermediate-risk FLT3-ITD patients with 1 of these 4 mutations forms a new subset of FLT3-ITD, intermediate-risk AML patients and this may have important implications for treatment decisions. In addition, patients who have the FLT3-ITD mutation without the addition of any of the 4 genetic abnormalities actually have a similar outcome to FLT3-ITD/CEBPa double-mutant patients.
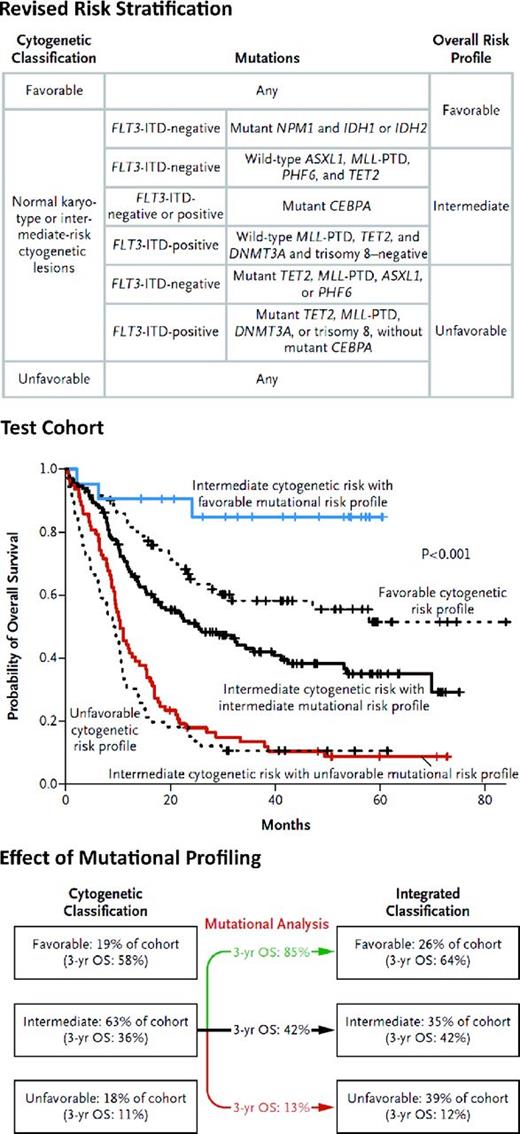
These data suggest that mutational profiling can be used to improve prognostication in AML, such that intermediate-risk patients can be reclassified as having favorable, intermediate, or poor risk based on the mutational status of 9 genes (Figure 2). This has important clinical implications, because patients with mutationally defined favorable risk have a better outcome with standard induction and consolidation than even patients with core binding factor–positive AML. In contrast, patients with mutationally defined adverse-risk AML have an outcome similar to patients with adverse karyotypic risk, and standard therapies are not sufficient to offer curative intent to the majority of these patients. We contend that future clinical trials should aim to identify genetically defined high-risk patients to offer these patients novel therapies early in their disease course, including novel therapeutic approaches to induction, consolidation, and maintenance therapy in an effort to reduce relapse and increase cure.
Revised risk stratification of patients with AML on the basis of integrated genetic analysis. Prognostic algorithm and survival curves with integrated mutational profiling are shown (used with permission from Patel et al21 ).
Revised risk stratification of patients with AML on the basis of integrated genetic analysis. Prognostic algorithm and survival curves with integrated mutational profiling are shown (used with permission from Patel et al21 ).
Although these studies that suggest mutational profiling offers significant added value to prognostication in AML, validation of these findings in other large, homogeneously treated patient cohorts is of utmost clinical importance. The findings from the E1900 trial were validated in an independent set of patients from the same cohort, but additional studies are needed to validate these results and extend them as additional disease alleles are identified in AML patients. In some cases, the results from different studies of specific genetic alterations has been inconsistent, including in the case of mutations in IDH1 and/or IDH2.33–35 However, data from the Medical Research Council trials in younger adults treated with aggressive therapy (induction followed by allogeneic or autologous transplantation) described favorable outcome with IDH2 R140 mutations or with double NPM1/IDH2 mutations,36 suggesting that cohort size, age, and treatment approach have significant effects on prognostication in AML. Therefore, subsequent biomarker efforts should determine whether different mutational genotypes predict outcome in large cohorts of younger and older adults treated with different AML therapies or if similar prognostic schemas can be used in different clinical contexts.
Anthracycline dose intensification for induction therapy in AML
The most widely used induction regimen for AML has remained the same for more than 30 years: 3 daily doses of daunorubicin (or its equivalent, anthracycline) at 45 mg/m2 and 7 days of cytarabine. As noted earlier, the ECOG E1900 trial evaluated the use of anthracycline dose intensification during induction therapy. A total of 657 patients between the ages of 17 and 60 years with de novo AML were randomized to receive either the standard 45 mg/m2 dose of daunorubicin or a higher dose of 90 mg/m2. The higher-dose cohort achieved a higher rate of complete remission and an increase in overall survival. The benefit of high-dose daunorubicin was restricted to those less than 50 years age of age and to those AML patients with cytogenetically defined favorable or intermediate risk. A subsequent study investigating high-dose versus low-dose daunorubicin in AML patients younger than 60 years of age reported findings similar to the ECOG E1900 trial.37 A similar study in patients older than 60 years38 observed an increased rate of complete remission in the higher-dose cohort (64% vs 54%). However, prolonged overall survival was only seen in patients under the age of 65 years and those with core-binding factor–positive leukemia.
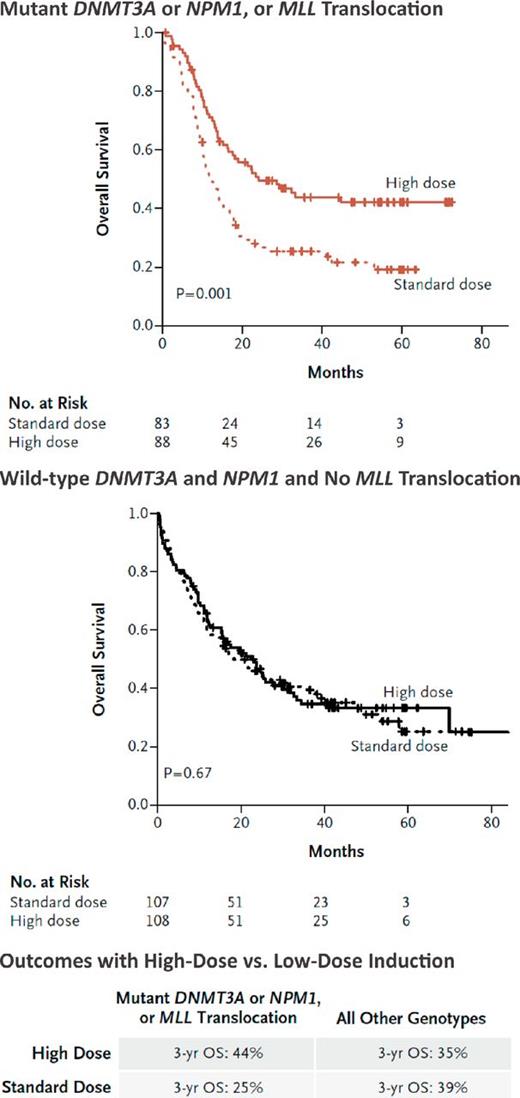
Unfortunately, these previous studies did not include prospective mutational profiling to identify biomarkers that could be used to delineate subsets of patients who would most benefit from intensified chemotherapy. This is particularly important given the heterogeneity in outcome in these clinical trials. A subsequent, post hoc analysis of mutational status, induction therapy, and outcome in the E1900 cohort revealed that high-dose daunorubicin improved outcomes markedly for patients with DNMT3A mutations.21 Patients with mutant DNMT3A receiving high-dose daunorubicin had similar outcome to DNMT3A wild-type patients receiving high-dose or standard-dose daunorubicin. Patients with MLL fusions or with NPM1 mutations also had improved overall survival when treated with high-dose daunorubicin (Figure 3). Interestingly, MLL fusions were mutually exclusive with DNMT3A and NPM1 mutations, suggesting a potential shared biologic mechanism explaining sensitivity to daunorubicin.
Molecular determinants of response to high-dose daunorubicin induction chemotherapy. Benefit of high-dose versus standard-dose daunorubicin in patients with NPM1/DNMT3A mutations or with MLL translocations compared with those wild-type for all 3 AML disease alleles is shown (used with permission from Patel et al21 ).
Molecular determinants of response to high-dose daunorubicin induction chemotherapy. Benefit of high-dose versus standard-dose daunorubicin in patients with NPM1/DNMT3A mutations or with MLL translocations compared with those wild-type for all 3 AML disease alleles is shown (used with permission from Patel et al21 ).
In total, patients with NPM1 or DNMT3A mutations or MLL translocations comprised 44.2% of the E1900 cohort; the 3-year overall survival for this group as a whole improved from 25% to 44% when the patients were treated with high-dose daunorubicin induction chemotherapy. A recent study suggested that patients with exon 23 mutations in DNMT3A had improved overall survival when treated with 12 mg/m2 of idarubicin (nearly equivalent to high-dose daunorubicin) versus daunorubicin,39 but these findings need to be further validated in a prospective clinical trial. These results suggest that mutational studies can be used to identify patients who benefit from dose-intense induction chemotherapy and provide an example of how molecular profiling can be used to decipher clinical heterogeneity in large, phase 3 AML trials.
Prognostic effect of novel genes in pediatric AML
Very few studies have assessed the prognostic value of recently identified genes in pediatric AML. A study of 460 pediatric patients with AML identified IDH1 and IDH2 mutations in 4% of this large cohort40 and IDH mutations were associated with improved overall survival in univariate analysis. Studies of the frequency of IDH1/2, TET2, and DNMT3A have shown that these mutations are rare compared with their frequency in adult AML,41 occurring in less than 5% of patients. For example, DNMT3A mutations have not been reported in pediatric AML, whereas approximately 22% of adult AML patients harbor DNMT3A mutations. These data suggest that pediatric AML may have markedly different genetic and epigenetic features compared with adult AML and may need to be studied as a separate entity to improve prognostication and to inform the biology of pediatric AML.
The evolution of relapsed AML
Despite an initial response to induction/consolidation therapy, most AML patients relapse with disease that is broadly resistant to chemotherapy. A recent study provided the first large-scale insight into the genetics of relapsed AML by performing whole-genome sequencing of 8 AML patients with relapsed AML.42 In each case, the AML genome at diagnosis and at relapse was sequenced and compared with genome sequencing of matched normal tissue. Instead of restricting themselves to somatic events in coding regions, the investigators validated all candidate somatic events in each tumor, allowing for a much greater number of mutations to be used to track the evolution from diagnosis to relapse. Two clear patterns emerged when the mutations found at diagnosis and at relapse were analyzed. In one model, a founding clone and multiple subclones clonally derived from this founding clone were extant at diagnosis. After chemotherapy, the residual cells from one of the minor subclones expanded to become the dominant clone at relapse. This clone also carried additional mutations that were only observed at relapse. Clonal progression from diagnosis to relapse of this nature was seen in 5 of the 8 patients in this study. In the other 3 patients in this study, the evolution from diagnosis to relapse was much simpler: the dominant clone at the time of diagnosis simply acquired more mutations at relapse. In all patients, there existed a founding clone that was not ablated by chemotherapy and was still persistent at relapse. Prospective identification of this clone at diagnosis would be of utmost clinical utility. The identification of mutations at diagnosis could serve as a tool for minimal residual disease measurement and allow for the deployment of therapies to eradicate residual clones after induction and consolidation therapy.
Translating novel genetic findings to the clinic
With the discovery of novel genes associated with AML pathogenesis continuing at a high speed, the challenge is to integrate this knowledge into the current clinical understanding of AML. Profiling each AML patient for clinically relevant and actionable lesions is the first step in this process. Although whole-genome and whole-exome sequencing have been critical technologies in cancer discovery efforts, their applicability today presents several challenges in the wider clinical setting. Although costs of sequencing are plummeting, the bioinformatics infrastructure and expertise needed to rapidly analyze sequencing data remain limiting in most settings. In addition, most of the mutational data in exome/genome studies represent passenger mutations and/or mutations without clinical or therapeutic relevance. Two recent proof-of-concept studies have demonstrated the feasibility of whole-genome sequencing in a specialized clinical setting. Welch et al43 used whole-genome sequencing to identify a cryptic PML-RARA translocation in a patient with clinical features of M3-AML that was not detected using FISH for PML-RARA. Roychowdhury et al44 performed whole-genome, whole-exome, and transcriptome sequencing on 2 patients with metastatic colorectal cancer and another with malignant melanoma. Even though groups at the cutting-edge of cancer genomics performed both of these studies, the studies still took 8 and 4 weeks to perform, respectively. Whole-genome sequencing will undoubtedly become part of the standard diagnostic evaluation within the next few years; however, the technology is currently not a feasible option for most AML patients at academic or nonacademic centers.
In contrast, the use of capture technologies followed by next-generation sequencing may represent the best option for clinical use of molecular genetic information in the near term. In this process, the relevant genes are “captured” by either PCR reactions performed in microdroplets45 or hybridized to oligonucleotide baits46 and then sequenced using next-generation sequencing. As newer “bench-top” sequencers are developed and optimized, their faster sequencing turnaround (1-3 days) will allow for high-throughput, albeit focused, mutational studies in the clinical setting so that these data can be used to inform induction and postremission therapies in AML.
Conclusion
With the discovery of new, recurrently mutated genes in AML, the first step should be to interrogate the clinical relevance of the specific mutation in homogeneously treated clinical cohorts in the context of all other known AML disease alleles. This will allow the AML field to evaluate the relevance of specific biomarkers rapidly and to develop tests that allow for cost-effective, rapid molecular profiling in the clinical setting. Whereas functional studies of novel disease alleles will likely result in the identification of novel therapeutic targets and lead to a greater understanding of AML pathogenesis, the incorporation of novel biomarkers into the clinical setting is the most important short-term goal facing AML patients and clinicians today.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Ross L. Levine, MD, Human Oncology and Pathogenesis Program, Leukemia Service, Department of Medicine, Memorial Sloan- Kettering Cancer Center, 1275 York Ave, Box 20, New York, NY 10065; Phone: 646-888-2767; Fax: 646-422-0856; e-mail: leviner@mskcc.org.