Abstract
Changes in the transplantation procedure and the implementation of effective supportive care strategies have decreased the incidence of infectious complications early after conditioning therapy for allogeneic hematopoietic stem cell transplantation (HCT) and have extended the duration of risks later. Therefore, the types of infections that cause significant morbidity and the timing of risks have changed. These late infections are caused by all types of organisms, bacterial, viral, and fungal, but risks are predictable and surmountable with the use of tailored prevention strategies. Specifically, recent studies document prolonged risks for bacterial infections in the setting of GVHD, especially those caused by encapsulated organisms and those secondary to impaired Ab responses. Both prophylaxis and vaccination strategies can be used as a means to prevent infections, which typically manifest in the respiratory tract. Multiple viruses cause infection later after HCT, including several herpesviruses (eg, CMV and varicella zoster virus) and other respiratory viruses such as influenza and adenovirus. These infections can cause severe disease with diagnostic challenges, but prevention strategies using enhanced monitoring and/or prophylaxis may be effective. Finally, fungi also cause disease late after HCT, especially filamentous fungi (eg, Aspergillus species and Mucormycoses) and Pneumocystis jiroveci; prophylactic strategies may be used successfully to prevent invasive infection. Late infections and methods to prevent them are reviewed herein.
Epidemiology and risks: general
Infections have constituted a major threat since the introduction of hematopoietic stem cell transplantation (HCT) more than 40 years ago. In fact, infections are a main obstacle to the success of HCT, along with relapsed malignancy and GVHD. The development of preventative regimens and strategies were introduced as tools became available. Some major improvements in outcomes were associated with application of drugs and molecular tests to detect and prevent early bacterial infections, HSV, CMV, and preengraftment candidal infections. In fact, a large review comparing more than 2500 patients who received allogeneic HCT in Seattle over 2 different time periods (1993-1997 vs 2003-2008) showed lower risks for death associated with infection over the latter years. Differences were notable in risks associated with CMV and those associated with gram-negative bacteria and fungi (both Candida species and molds).1 We have made strides in preventing these infections, largely due to more aggressive prophylaxis strategies that use quinolone antibiotics and fluconazole and early screening strategies using molecular methods and radiology to detect and prevent CMV infection from causing end-organ disease.
Although our strategies have decreased the impact of early infections, limitations in preventative strategies and changes in transplantation methods now favor the development of later infections after HCT. Drug toxicities and limitations in molecular screening methods do not allow for effective application in some outpatient arenas. Changes in hosts and conditioning regimens that have reduced toxicity but extended durations of GVHD have effectively altered the predicted epidemiology of infection, with risks now occurring later after engraftment. Similarly, the use of alternative stem cell products such as peripheral blood rather than BM may be associated with later risks for infection during the GVHD period. Unfortunately, many analyses only provide a glimpse of actual outcomes, reporting infectious complications as a larger, nonspecific variable, transplantation-related mortality. Therefore, our knowledge on infectious risks has been generated largely from single-center retrospective cohort studies and from adjunctive evaluations of randomized trials. Several such studies have now documented the scope of “late risks.” For example, one study that evaluated infectious complications associated with the use of peripheral blood stem cells compared with BM transplantation (BMT), suggested that recipients of peripheral blood stem cells have shorter durations of neutropenia but higher risks of postengraftment infections, and, accordingly, no difference in the use of antibacterial, antifungal, or anti-Pneumocystis prophylaxis.2 Analyses also suggest that the increased use of reduced-intensity conditioning (RIC) transplantations may favor the development of later infections. Several cohort analyses and case-control studies have emphasized persistent infectious morbidity late after RIC; however, because specific risks are different during these time periods, the epidemiology of infection and sometimes outcomes also differ.3 Finally, the type of prophylaxis and treatment for late complications such as GVHD likely has a large impact on risks for late infections, although few comparative studies have been performed. One retrospective study demonstrated that the dose of corticosteroids used for initial treatment also affects subsequent infection risks, with low-dose prednisone equivalents (≤ 1 mg/kg/d) being associated with lower risks for fungal infections and mortality.4
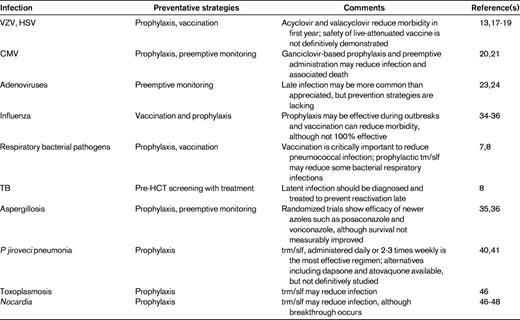
Although infectious risks persist late after HCT, the timing of infection is unpredictable and multiple variables affect the likelihood of infection. Therefore, surveillance strategies and prophylaxis regimens should be tailored according to clinical risks. However, with an understanding of immunopathogenesis and risk-benefit ratios, these risks present a surmountable challenge and effective preventative strategies can be used. The most common infections and prevention strategies are summarized in Table 1 and discussed in detail in the following sections.
Bacterial infections
Large population-based studies have shown that the spectrum of bacterial infections has changed over time, with a notable shift from gram-negative bacteria causing bloodstream infection to gram-positive organisms as a primary cause of disease. This is thought to be due to prevention regimens and maintenance of prolonged intravascular catheters. The center-based studies have failed to demonstrate how changes in transplantation modalities have affected the epidemiology of bacteremia. Specifically, several case-control studies have documented equivalent or higher numbers of bacteremias during the postengraftment period after RIC, but a shift in the types of organisms favoring typical catheter-acquired gram-positive bacteria late after RIC rather than the gram-negative Enterobacteriaceae that are typically gut-acquired after myeloablative conditioning.3,5,6 Specific bacterial infections that are common late after HCT are worthy of detailed discussion.
Streptococcus pneumoniae
A major risk during the late transplantation period is respiratory acquisition of “pneumonia pathogens.” During the late period of poor Ab and cellular immunity, encapsulated bacteria such as S pneumoniae can cause the rapid development of pneumonia and/or meningitis with the potential for high morbidity. Both prophylaxis with trimethoprim-sulfamethoxazole and Pneumococcus vaccinations are at least partially effective in preventing disease. A review from the M.D. Anderson Cancer Center summarized S pneumoniae infections during the long period from 1989-2005. During this time, the calculated incidence of infection was 7 per 1000 HCTs performed. The infection typically did occur late, at a median day of diagnosis of 443 days, with underlying lymphoma and receipt of corticosteroids playing a role in increasing risks.7 Another population-based surveillance study performed in Toronto in 1994-2005 documented that the risk for S pneumoniae infections in HCT recipients was 347 per 100 000 person-years compared with 11.5 per 100 000 person-years in the general population.8 Results of this study emphasized the limitations of our prevention strategies, because the major serotypes that caused disease would typically have been protected by the available vaccine, which was not given in many people. Although trimethoprim-sulfamethoxazole can prevent some infections, this center also reported high rates of drug resistance in infecting isolates, further emphasizing the importance of timely vaccination.
Antibiotic-resistant bacteria
Much has been written about the morbidity of the increasingly complicated multidrug-resistant (MDR) bacteria, both gram-positive and gram-negative. Organisms such as vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and MDR Enterobacteriaceae (ESBL and KPC-producing gram-negatives) have become problematic at variable frequencies in different transplantation centers. These organisms can be acquired through the gastrointestinal (GI) tract early after HCT and later through multiple different routes, especially in people in whom endogenous flora have been altered due to prolonged or recurrent antibiotic exposure.
Risks for MDR organisms are important to understand given the implications on initiation of effective empirical antibiotic therapies. One large multicenter study performed in Brazil documented the scope of these pathogens. Prospective surveillance performed among 13 centers between March and November 2004 documented bacteremia in 91 of 411 patients (27%).9 Thirty-seven percent of these infections were caused by MDR pathogens, which were defined as those resistant to at least 2 classes. This study did show clear clustering in specific hospitals and specific host risks, including cumulative exposure to third-generation cephalosporins. Although these infections may not be entirely preventable during the late-HCT period, risks are important to understand to choose optimal first-line therapies in febrile patients. Centers that have high rates of MDR gram-negative bacterial infections should consider increasing vigilance toward screening for colonization to predict breakthrough infection and to enhance infection control.
One pathogen that is important to consider is P aeruginosa, which is particularly common as a cause of pneumonia later after HCT. In a review performed at the Fred Hutchinson Cancer Research Center in Seattle between 1990 and 2001, 95 of 5772 patients (1.7%) developed Pseudomonas pneumonia.10 The majority of cases occurred late during GVHD at a median of 63 days after HCT (range, 5-1435). Results of this review are important because they emphasize the need for the establishment of microbial diagnosis, with high risks of copathogens and recurrent Pseudomonas pneumonia associated with short-course antibacterial regimens.
Clostridium difficile colitis
Although we have classically considered C difficile colitis to be a healthcare-acquired infection that complicates early hospitalization, the results of 2 recent studies in the HCT population have emphasized the importance of late disease.11,12 These 2 large, single-center cohort studies documented that approximately half of cases occur later (greater than 1 month) after HCT and largely in association with GVHD. Although GVHD was noted to play a role as a risk in either study, results also suggested that C difficile colitis may actually serve to increase the likelihood of subsequent GVHD involving the GI tract.11 More studies are needed to understand the significance and to devise more effective prevention strategies for C difficile colitis.
Mycobacterial infections
Both Mycobacterium tuberculosis and atypical (rapid and slow growing) mycobacteria are a significant cause of disease late after HCT, especially in people with poor T-cell immunity. Rates of reactivation tuberculosis (TB) after HCT have historically been reported to be low, but rates are variable according to endemicity and pre-HCT seropositivity. However, many centers now observe devastating outcomes of previously unrecognized latent pulmonary TB in association with increased rates in certain endemic areas or transplantation of people from endemic regions.8 Discussion of how to prevent this occurrence is ongoing. Because recognition of latent infection based on skin test positivity has relatively poor sensitivity in people with hematologic malignancies, several investigators suggest enhanced pre-HCT screening of people at high risk using more sensitive methods of testing (eg, IFN-gamma release assays) in adjunct to the purified protein derivative skin test. Recognition of latent infection allows for the administration of effective treatment, which is especially important in people who are projected to have considerable GVHD or delayed T-cell engraftment.
Mycobacteria other than M tuberculosis, both rapid- and slow-growing organisms, cause disease late after HCT, especially infections involving the lungs and/or bloodstream. Prevention of these infections is difficult, but recognition with typical signs and symptoms is critical given the need for alternative and prolonged antimicrobial regimens.
Viral infections
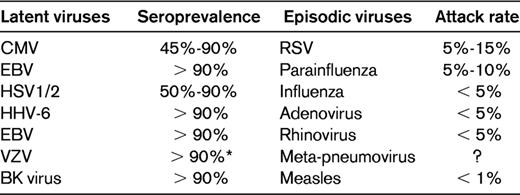
Viruses that cause infection after HCT can be classified as typically latent or “episodic” in nature, with the latter acquired typically after exposure rather than as a result of a reactivation event (Table 2). It is important to understand the difference in viral pathogenesis because it has an impact on the type of preventative strategy that one would employ. Screening for reactivation with preemptive treatment and application of prophylaxis in seropositive recipients plays a role in preventing disease caused by latent herpesviruses such as CMV, HHV-6, and varicella zoster virus (VZV). Vaccinations have been developed to enhance protective responses to infection caused by episodic viruses such as influenza. Select viruses and prevention strategies are discussed in the following sections.
HSV and VZV
Reactivation HSV-1 and HSV-2 can occur both early and late after HCT, but the actual risk has been decreased dramatically by the generous use of acyclovir prophylaxis after allogeneic HCT. Acyclovir resistance has been described in severely T cell–depressed patients who have had prolonged or recurrent prior exposure, although it does not appear to be common. One study that summarized HSV disease in 3 retrospective cohorts of HCT recipients found that the 2-year probability of infection estimates were 30% in people who did not receive acyclovir prophylaxis beyond day 30, as opposed to 0%-4% when the drug was continued longer. Acyclovir resistance was not documented frequently in this study.13 A more recent study that used PCR to identify acyclovir-resistant HSV-1 secreted in the setting of oral ulceration and mucositis suggested that the actual prevalence of acyclovir-resistant latent HSV is higher than previously understood.14 It is likely that variability in studies relates to severity and chronicity of T-cell suppression and dosing of prophylactic acyclovir.
VZV causes a large amount of morbidity late after HCT, with risks for both dermatomal reactivation and disseminated disease. Severe, potentially fatal disease, including hepatitis and meningoencephalitis, can occur late after HCT even in the absence of skin lesions.15 Early studies demonstrated that allogeneic HCT recipients have a high risk for VZV reactivation, especially later after transplantation (> 3 months) in the setting of GVHD.16 More recent studies suggest that prolonged acyclovir prophylaxis effectively decreases VZV-related morbidity.17,18 Results of these studies have propelled many centers to administer acyclovir for the first year after allogeneic HCT. There is some controversy over the safety of the live, attenuated vaccine in this setting, although there have been reports of success and apparent safety using this method.19
CMV
CMV infection is common in the general population, and seropositivity in the donor and/or a HCT recipient is the norm. Reactivation of virus, potentially resulting in organ disease, causes morbidity and death if not managed appropriately. Prevention strategies have shown that prophylaxis with ganciclovir and preemptive therapy based on viral detection (using either antigen or PCR) decreases the risk for disease effectively, with some limitations to both strategies. However, the use of antiviral therapy, and prolonged T-cell engraftment has delayed CMV-specific protective immunity so that late infection is the norm. In one study, late CMV disease developed in 17.8% of patients at a median of 169 days after HCT (range, 96-784). There is some hint that late disease may cause more variable clinical syndromes, with GI tract disease, retinitis, and meningoencephalitis described more frequently during this late period.20,21 How to prevent disease late after HCT is a matter of debate because this is a time period during which screening for viral reactivation and prophylactic antivirals may be difficult. Studies have shown that acyclovir compounds at high doses may reduce CMV infection, but not disease after allogeneic HCT.22,23 Ganciclovir reduces CMV infection and disease, but has had no effect on overall observed survival, possibly because of BM toxicities.22,23 A recent study evaluating a lower dose of maribavir failed to show superiority compared with placebo, but that study appeared to be underpowered.24 More efforts are needed to define how to prevent this disease late after HCT.
Adenovirus
Severe disease caused by disseminated adenovirus is associated with poor T-cell reconstitution and GVHD. Disease can mimic GVHD, with onset of fever, diarrhea, hepatitis, and rash. How to prevent this disease remains unclear because antivirals do not have great activity and preemptive screening may enable decreased immunosuppression (and prevention of disseminated disease) in only a handful of people.25,26
Polyomaviruses
BK virus and John Cunningham (JC) virus are members of the polyomavirus family that are increasingly being recognized as a cause of human disease. JC virus is neurotropic and associated with progressive multifocal leukoencephalopathy, and BK virus is increasingly being recognized as a cause of hemorrhagic cystitis.27 Because BK virus is present in approximately 90% of the adult population as a latent virus in the uroepithelium, there has been some question regarding how to effectively diagnose and prevent hemorrhagic disease. GVHD presents a risk for BK virus–associated hemorrhagic cystitis. Some have proposed that conditioning-related damage to the uroepithelium allows for viral replication, which drives a cycle that becomes unchecked and exacerbated by immune reconstitution and alloreactivity.28 Although BMT patients with hemorrhagic cystitis frequently have measurable viruria and even viremia before active cystitis, definitive prevention strategies have not been developed in therapeutic trials.29,30
Influenza and other episodic viruses
Risks for upper respiratory infections caused by episodic viruses in HCT patients are equivalent to those of the population as a whole; however, these patients have increased susceptibility to lower tract disease, including bronchiolitis, pneumonitis, resultant lung damage, and secondary infection. Mortality associated with viruses such as influenza, RSV, and parainfluenza may be quite high, and infection control complications may be exaggerated by prolonged viral shedding in BMT recipients.31–33 Unfortunately, few methods have been shown to prevent infection with viruses other than influenza. Prevention of influenza infection is enabled by vaccination, which, although not 100% effective, should be administered routinely; in outbreak settings, prophylaxis with neuraminidase inhibitors may be considered for high-risk HCT recipients.34–36
Fungal infections
Recognition of morbidity associated with filamentous fungal infections involving the lungs has triggered increased efforts to develop methods for preventing infection late after allogeneic HCT. In the 1990s, epidemiologic studies showed that the median day of invasive pulmonary aspergillosis was approximately 90 days (or greater) after allogeneic HCT. Prophylaxis studies were then performed to evaluate mold-active azole drugs (ie, itraconazole, posaconazole, and voriconazole) compared with placebo and lipid formulations of amphotericin B using different dosing algorithms. The most promising results have been with the newer azole formulations posaconazole and voriconazole, which both decrease the risk for invasive aspergillosis compared with placebo.37,38 However, none of these studies have demonstrated improved survival, creating controversy over the utility of administration. Screening methods for Aspergillus infection using enzyme immunoassays that detect galactomannan antigen are also available. Although studies have shown that this test can be positive before the onset of clinical signs and symptoms of disease, the utility of screening with preemptive therapy has not been evaluated in a trial powered to test infection or survival. A recent randomized trial performed in multiple centers in Australia showed that preemptive antifungal therapy applied using the combination of PCR and galactomannan detection allowed for decreased use of antifungal therapy administered empirically for fever.39 However, other fungi cause disease during this late time period, especially agents of mucormycosis, which almost exclusively occur late after allogeneic HCT and may warrant alternative antifungal therapies.
Pneumocystis is another fungus that typically occurs late after HCT as a cause of pneumonitis. Effective prevention strategies are available, and the most effective option is trimethoprim sulfamethoxazole (tm/slf), which can prevent Pneumocystis pneumonia effectively after daily administration or even staggered administration 2-3 times weekly.40,41 Alternatives to tm/slf include atovaquone, dapsone, and aerosolized pentamidine. Results of multiple studies suggest that the efficacy of dapsone is inferior compared with tm/slf, with frequent breakthrough infection and toxicity such as methemoglobinemia and anemia.42,43 No randomized trial has definitively compared the efficacy of atovaquone in this population, and this drug is limited by GI side effects and costs. Generally speaking, tm/slf is the mainstay prevention for the prevention of fungal infection after HCT, although P jiroveci isolates with resistance to the drug due to mutations in the target enzyme gene dihydropteroate synthase have been shown to be a cause of disease in various populations studied.44,45 It is also important to emphasize the other “secondary” benefits associated with tm/slf prophylaxis, because this drug may effectively prevent toxoplasmosis, pulmonary Nocardia infections, and infection with other susceptible organisms such as Pneumococcus.46–48
Conclusions
The bulk of infectious morbidity and mortality in allogeneic HCT recipients is now focused on the late, postengraftment time period largely due to successful early prevention strategies and changes in transplantation methods. However, bacterial, viral, and fungal infections are common and account for a still unacceptable number of delayed deaths. Fortunately, the prevention of many infections is possible because of well-tolerated prophylaxis regimens, enhanced screening strategies, and vaccination. Although none of our current strategies is “bullet-proof,” a systematic approach positions delayed infections as a surmountable challenge.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from and consulted for Astellas, Merck, and Pfizer. Off-label drug use: None disclosed.
Correspondence
Kieren A. Marr, MD, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross 1064, Baltimore, MD 20105; Phone: 410-614-9141; Fax: 410-614-0714; e-mail: Kmarr4@jhmi.edu.