Abstract
The outcome of allogeneic stem cell transplantation has improved over the past decades due to a significant reduction of nonrelapse mortality, whereas our ability to control underlying malignant diseases has remained unchanged. Reduction of nonrelapse mortality has been achieved in matched sibling donor transplantation, but perhaps more so with unrelated donor transplantation, in part due to the advances in HLA matching between donor and recipient, but also as a result of improved supportive care, better GVHD prophylaxis, and tailored conditioning regimens. Therefore, over the past decade, results of matched sibling donor and unrelated donor grafts have grown more similar, and the difference in 1-year survival for patients with leukemia has gone from 21% in 1988 in favor of MSD to 9% in 2008. However, due to the significant and combined effect of patient, transplantation, and donor variables, comparisons are made here in the context of defined subsets of patients and specific diseases and in some circumstances also looking at separate studies in children and adults.
Introduction
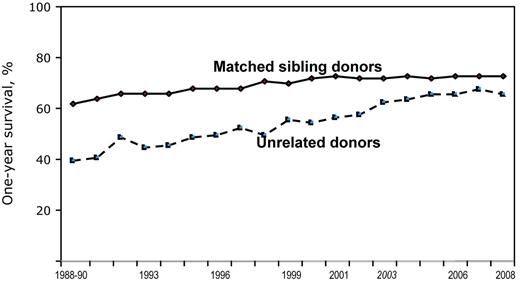
Unrelated donor (UD) stem cell transplantation (SCT) has been growing steadily over the past decades, suggesting increasing success rates in patients with malignant and nonmalignant hematologic disorders and in children with inborn errors of metabolism or combined immune deficiency. As a result, the difference between matched sibling donor (MSD) and UD transplants has been steadily declining (Figure 1). In the meantime, the concept of “matching” for UD transplantations has also evolved significantly. Today, we would consider a matched UD to be an HLA allelic identity at the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci, which is referred to as an 8/8 match. Further refinement of the concept of matching may include matching at the HLA-DQ and HLA-DP loci.1 For the purposes of this review, 3 categories of donors will be considered: (1) 8/8 matched UD, (2) mismatched UD (< 8/8 unless otherwise specified), and (3) MSD transplantations, who are by definition genotypically HLA matched (note that not all studies reported in this review specified whether 8/8 referred to antigen or allele matching).
Outcomes by year, sibling versus unrelated: 1-year survival after myeloablative conditioning for acute leukemias in any remission phase, CML or MDS, age < 50 years, by year of transplant and graft source, 1988-2008. One-year survival rates after transplantation have generally improved over the past 2 decades. Outcomes of unrelated donor transplants are approaching the rates of related donor transplants. Overall survival rates at 1 year are 74% (related donor) and 65% (unrelated donor) for these transplants in 2008. Improvements in HLA-matching techniques with consequently better donor selection, better overall patient selection for transplantation, and improvements in supportive care are the likely explanations for this trend. CIBMTR data used with permission.
Outcomes by year, sibling versus unrelated: 1-year survival after myeloablative conditioning for acute leukemias in any remission phase, CML or MDS, age < 50 years, by year of transplant and graft source, 1988-2008. One-year survival rates after transplantation have generally improved over the past 2 decades. Outcomes of unrelated donor transplants are approaching the rates of related donor transplants. Overall survival rates at 1 year are 74% (related donor) and 65% (unrelated donor) for these transplants in 2008. Improvements in HLA-matching techniques with consequently better donor selection, better overall patient selection for transplantation, and improvements in supportive care are the likely explanations for this trend. CIBMTR data used with permission.
The role of some crucial components of SCT, such as the intensity of the conditioning, the type of GVHD prophylaxis, and the stem cell source, as they relate to donor type will be examined first.
Intensity of the conditioning regimen
The intensity of conditioning regimens can vary significantly, from nonmyeloablative (NMA) conditioning to reduced intensity conditioning (RIC) to myeloablative (MA) conditioning.2 A recent study by the Center for International Blood and Marrow Transplant Research (CIBMTR) addresses the combined effect of conditioning intensity, radiation, stem cell source, donor type, and GVHD prophylaxis primarily on GVHD, but also on survival.3 The investigators divided the patients in 6 separate categories: (1) MA with total body irradiation (TBI) plus peripheral blood stem cells (PBSCs; n = 700), (2) MA + TBI + BM (n = 245), (3) MA + non-TBI + PBSCs (n = 1017), (4) MA + non-TBI + BM (n = 492), (5) RIC + PBSCs (n = 622), and (6) RIC + BM (n = 67). The reference group was MA + TBI + PBSCs; the groups with the lowest comparative risk of acute GVHD were the MA conditioning + non-TBI + BM, both for MSD and UD transplantations; tacrolimus was also associated with less GVHD. Other significant predictors of outcome were female donors in male recipients, which were associated with more nonrelapse mortality (NRM) and higher risk of severe acute GVHD.3 These results suggest that conditioning intensity, graft source, and TBI have a combined effect on transplantation outcome, which needs to be considered in deciding on treatment strategy. For example, if the conditioning is MA and TBI is given, it may be reasonable to use BM and not PBSCs; the contrary would be true for RIC regimens. Matching for HLA has an independent effect in the UD cohort. When looking at donor type, NRM at 5 years was 31% for MSD and 40% for UD grafts, with actuarial overall survival (OS) of 46% and 33%, thus suggesting an overall negative effect of using a UD.
T-cell Abs in allogeneic HSCT
GVHD prophylaxis is another crucial component of the transplantation protocol, and several T-cell Abs are available for clinical use, including antithymocyte globulin (ATG) from horse or rabbit and mAbs, of which alemtuzumab (also known as “CAMPATH ” for “Cambridge Pathology,” where it was developed by H. Waldman), is the most widely used. The question is whether the addition of ATG or CAMPATH to a conventional transplantation regimen is beneficial. To answer this question, 2 prospective randomized trials testing ATG in UD grafts and 1 large registry-based study including ATG and CAMPATH in both UD and MSD grafts are discussed.
Randomized trials
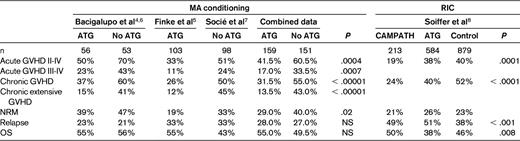
Table 1 summarizes results from 2 prospective randomized trials that enrolled 310 patients after MA conditioning regimens; both have been published with a short follow-up4,5 and both have been updated to better assess chronic GVHD.6,7 Patients receiving ATG had 20% less acute grade II-IV GVHD (P = .0004), 15% less grade III-IV GVHD (P = .0007), 20% less chronic GVHD (P < .0001) and 30% less extensive chronic GVHD (P < .00001). Relapse and survival were comparable in patients receiving or not receiving ATG (Table 1). More patients in the ATG group could discontinue immunosuppressive therapy early, had Karnovsky scores > 90, and were less likely to have chronic lung dysfunction and late NRM.6,7
Registry-based study
A recent study investigated the effect of in vivo T-cell depletion (TCD) on MSD (n = 792) or UD (n = 884) transplantations. All patients received RIC; 584 also received ATG, 213 CAMPATH, and 879 received no Abs.8 Patients receiving CAMPATH had the lowest risk of acute and chronic GVHD, followed by ATG patients, whereas patients not receiving Abs had a higher risk. However, relapse risk was high in patients receiving T-cell Abs and disease-free survival (DFS) was higher in patients not receiving T-cell Abs (Table 1). The results of this study is consistent with randomized trials showing reduced GVHD, but suggests higher relapse risk and lower DFS. The investigators of this study themselves point to the weakness of this registry based study: “the major limitation is that choice of treatment strategy, including whether or not to use in vivo TCD, was at the discretion of the transplantation center, and therefore subject to bias.”8 An additional reason for different results compared with the prospective trials is the intensity of the conditioning regimens: RIC versus MA conditioning. Therefore, the question of whether T-cell Abs are beneficial may be the wrong question if not placed within the context of a given transplantation setting.
The registry-based study was repeated by the CIBMTR in 715 children and adolescents with acute lymphoblastic leukemia (ALL) grafted from a UD between 1998 and 2007, comparing the use of T-cell Abs (ATG and CAMPATH) with T cell–replete grafts9 (Table 1). CAMPATH and ATG reduced acute and chronic GVHD significantly and had no negative impact on relapse; 5-year OS was best with CAMPATH (55%), followed by T cell–replete and ATG grafts (both 45%).
Ex vivo TCD
One study showed that changing the method of TCD to ex vivo may provide different results compared with the use of in vivo TCD with Abs (Table 1).10 That study prospectively randomized patients to receive a T cell–replete graft or a graft depleted of T cells with different methods. There was a reduced risk of acute GVHD for patients receiving TCD grafts, but no difference in chronic GVHD and worse outcome compared with patients receiving T cell–replete grafts due to a higher rate of relapse in TCD patients.10 There was no selection bias in that study, because patients were prospectively assigned or not to TCD, so it is unclear why the ATG “in vivo TCD” prospective trials and this “ex vivo TCD” study yielded different results, especially regarding chronic GVHD and outcome. One reason may be that we do not know which T cells are beneficial for GVL and which are detrimental for GVHD and mortality. Again the answer to the question of whether one should use TCD or not cannot be answered simply by yes or no; it probably depends on other risk factors for GVHD. In a setting of high GVHD risk (ie, MA conditioning, TBI, PBSC, UD),3 some form of TCD may be beneficial. In a situation of low risk of GVHD, TCD may actually be detrimental. Indeed, one should not forget the negative effect of T-cell Abs on immune reconstitution, which will increase the risk of infections, as documented for EBV, CMV, adenovirus, and other potentially lethal infections.10 In addition, the reduction of GVHD has usually been associated with an increased risk of relapse of the original disease, and this may be particularly true in patients with advanced disease.
The role of HLA matching
In 2007, the CIBMTR and National Marrow Donor Program (NMDP) reported on the role of HLA matching in UD transplantations.11 A single mismatch detected by low- or high-resolution DNA testing at HLA-A, HLA-B, HLA-C, or HLA-DRB1 (7/8 match) was associated with higher mortality (relative risk [RR] = 1.25; P < .001) and a 1-year survival of 43% compared with 52% for 8/8 matched pairs. Single mismatches at HLA-B or HLA-C appear to be better tolerated than mismatches at HLA-A or HLA-DRB1. Mismatching at 2 or more loci compounded the risk. The effect was most profound in patients with early disease, in whom each HLA mismatch decreased survival by 10%; it was less clear in patients with intermediate or advanced disease. The conclusions are that patient factors such as phase of the disease remain critical predictors of survival: when the disease is advanced, identifying a fully matched donor may not improve survival significantly and must be balanced against the risk that the disease will progress while a prolonged search is ongoing.
The search for an optimal donor has gone beyond the HLA system to include killer cell Ig–like receptor (KIR) haplotype typing, which has shown a protective effect against relapse.12 AML patients grafted from Cen-B homozygous UDs have a 16% cumulative incidence of relapse, compared with 37% for patients grafted from Cen- A/A donors (P < .001). A role for killer cell Ig–like receptor genotype of the donor on GVL has also been identified in MSD transplantations.13
The role of donor age
In 2001, the NMDP reported that donor age was an independent risk factor for GVHD, DFS, and OS after UD transplantations.14 The OS at 5 years was 33%, 29%, and 25% for donors 18-30, 31-45, and more than 45 years of age, respectively (unpublished EBMT 2012 data). A recent European Group for Blood and Marrow Transplantation (EBMT) study reported on 719 myelodysplastic syndrome (MDS) patients over the age of 50 years who had been grafted from an MSD (n = 555) or an UD (n = 164; Table 2). The median age of UDs or MSDs was 34 and 56 years, respectively. There was no effect of donor age in the 555 MSD patients, whereas a strong effect of donor age was seen in UD patients. The adjusted 5-year OS was over 50% for UD transplantations from donors < 30 years of age and 30% for all MSD grafts and for UD transplantations from donors older than 30 years. The conclusion of this study was that a patient over the age of 50 years with MDS, if given the choice, should have an allogeneic SCT from a young UD rather than from an older MSD.
SAA
In patients with acquired severe aplastic anemia (SAA), the overall outcome of UD versus MSD transplantations in the last decade (1999-2009) shows a survival advantage for MSD (76% vs 58%, P < .001) in univariate analysis. The advantage of MSD, however, became smaller (11%) in the last 5-year interval (2005-2009) compared with 18% in first quinquennium (1999-2004), suggesting improved outcome for UD transplantation in the more recent cohort. In multivariate analysis, including stem cell source, interval diagnosis transplantation, patient age, and use of ATG in the conditioning regimen, donor type (MSD vs UD) is not a significant predictor of outcome. These results are consistent with the excellent results reported recently by several groups using UD for allografts in acquired SAA.15–17 All of these studies seem to be based on a conditioning including cyclophosphamide and fludarabine (FC) with ATG (FCA) or CAMPATH (FCC): the FCA regimen seems to require low-dose TBI in adults to allow consistent engraftment,16 whereas FCC appears to be applicable without TBI. In the 2 studies using FCC, this was true for both children and adults; a conditioning regimen without TBI would be desirable for nonmalignant disease.17,18 In summary, UD transplantations are proving to be an excellent treatment strategy for patients with SAA, and outcome in recent years is not statistically different compared with MSD transplantations. This may be relevant for patients who have failed a first course of immunosuppressive therapy and may also be considered for frontline therapy in selected cases.18
FA
Allogeneic SCT for Fanconi anemia (FA) from a MSD is currently associated with an astonishing 93% OS20 ; this result is achieved with low-dose cyclophosphamide as the sole conditioning regimen. The outcome of UD grafts has been less encouraging, with significant rates of GVHD. Guardiola for the EMBT reported 69 FA patients undergoing UD transplantation in 2000, with 83% sustained engraftment and 33% OS at 3 years.19 Wagner for the CIBMTR reported 98 FA patients in 200720 with 89% engraftment when fludarabine was part of the conditioning compared with 69% for no fludarabine; the 3-year OS was 52% vs 13% respectively for fludarabine compared with no-fludarabine regimens.
The EBMT recently analyzed 839 FA patients grafted between 1972 and 2009 from a MSD (n = 544) or a UD (n = 295; Table 3). The OS at 10 years is 60%. For patients grafted before the year 2000, MSD showed better OS at 10 years compared with UD (65% vs 45%); for patients grafted beyond 2000, the 10-year OS has improved to 80% for MSD and to 66% for UD transplantations.21 An MSD remains the donor of choice, although there has been significant improvement with UD transplantations, and results are less discrepant in the more recent transplantation era.
Other pediatric indications for SCT
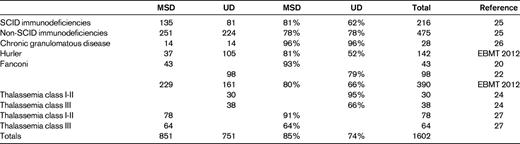
Other pediatric indications, including primary immune deficiencies, SCID and non-SCID, chronic granulomatous disease (CGD), Hurler disease, and thalassemia, and the FA studies, are listed in Table 3: studies comparing MSD or UD are reported for more than 2000 patients. On average, there is a survival advantage for MSD transplantations in terms of 3-year OS (82%) compared with UD transplantations (72%). However, in some indications, the outcome is very close (unpublished EBMT 2012 data).22–24
ALL
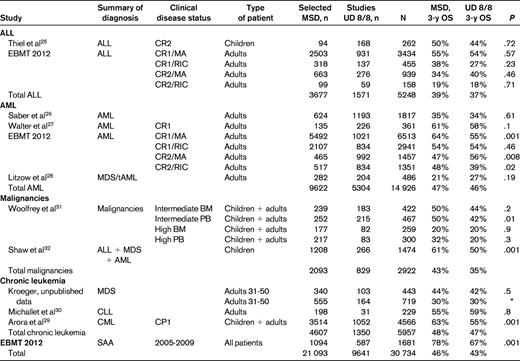
In the pediatric age group, leukemia-free survival (LFS) after MSD or UD transplantations was reported to be comparable by the CIBMTR.25 In that analysis, the 3-year actuarial LFS was 50% for MSD, 44% for matched UD BM transplantations, and 44% for mismatched UD BM transplantations. There was a trend for increased NRM in mismatched UD (P = .04) compared with MSD, but relapse and overall mortality were the same in the 3 groups (Table 2).
An EBMT study has looked at more than 6000 adults with acute lymphoblastic leukemia (ALL) in first complete remission (CR1) receiving a MA conditioning regimen: the 5-year OS was 55% and 54% (P = .57) for MSD and UD, respectively (Table 2). A comparable outcome was seen in ALL CR1 patients receiving RIC, with 54% OS in both groups (P = 0.23). In adults with ALL CR2 given MA conditioning, OS was superior, although not significantly so, with UD (40% OS) versus MSD (34%, P = 0.46). In patients with ALL CR2 prepared with RIC regimens, OS was similar for MSD and UD grafts (OS 19% vs 18%, P = .71), but significantly worse compared with MA regimens.
These studies are summarized in Table 2 and comprise over 5 000 ALL patients: the 3-year OS is 39% for MSD and 37% for UD transplantations.
AML
The CIBMTR study on acute myeloid leukemia (AML) has been published recently26 : 624 patients were grafted from MSD, 1193 from an 8/8 matched UD, and 406 from a 7/8 UD. The 100-day incidence of grades B-D acute GVHD was significantly lower in MSD SCT recipients compared with 8/8 UD and 7/8 UD SCT recipients (33%, 51%, and 53%, respectively; P < .001). In multivariate analysis, 8/8 UD SCT recipients had a similar survival rate (35%) compared with MSD HCT recipients (34%; RR =1.03; P = .62). Patients receiving a 7/8 UD SCT had higher early mortality than MSD SCT recipients (RR = 1.40; P < .001), but beyond 6 months after SCT, their survival rates were similar (RR = .88; P = .3; Table 2). This CIBMTR study suggests similar long-term outcomes in AML patients receiving transplantation from MSD or UD, whether 8/8 or mismatched for 1 HLA antigen (7/8).
In a univariate analysis on a large number of patients, results from the EBMT Acute Leukemia Working Party are also reported in Table 2: the LFS at 3 years is superior for MSD (56%) compared with UD 8/8 (47%) after MA conditioning, but it is comparable after a RIC regimen (54% in both groups). When AML is grafted in CR2, the outcome seems significantly superior with an 8/8 UD after MA conditioning (56% vs 47%, P = .008); the opposite is true after a RIC regimen, with MSD having superior LFS (Table 2).
Another study has addressed the role of the donor in patients with AML in CR1: 91 patients were grafted from UD and 135 from MSD. The 5-year estimates of OS were 58%. The outcome was not statistically different for MSD and UD (Table 2). Overall mortality among 9/10 and 10/10 URD recipients was also similar (adjusted hazard ratio = 1.16; unpublished EBMT 2011 data).24
All of these studies are summarized in Table 2 and include more than 14 000 patients with AML in different phases of their disease: the average 3-year OS is 47% for MSD and 46% for UD transplantations. The small difference is consistent with single studies.
Chronic leukemias
We have already discussed the role of donor age in the context of an allogeneic SCT for elderly patients with MDS. MDS patients receiving a transplantation from a young UD fared better than MDS patients receiving transplantation from older matched siblings (unpublished EBMT 2012 data; Table 2). Similarly, in a CIBMTR study, 8/8 matched UD transplantations had superior survival compared with MSD transplantations.28 Chronic myeloid leukemia is no longer a popular indication for an allogeneic SCT; however, in a recent CIBMTR-based study of 4566 chronic myeloid leukemia patients grafted in first chronic phase, the 3-year OS was significantly superior for MSD transplantations (63%) compared with UD transplantations (55%29 ; Table 2). A study led by Michallet on behalf of EBMT investigated 229 patients with chronic lymphocytic leukemia (Table 2)30 : the 3-year OS was 55% versus 57% for MSD and UD grafts, respectively (P = .8).
In a study on different hematologic malignancies31 including both children and adults, the CIBMTR compared 8/8 allelic matched UD with MSD transplantations (Table 2). In multivariate analysis, recipients of MSD transplantations had less transplantation-related mortality, acute GVHD, and chronic GVHD, along with better DFS and OS, than transplantations from 8/8 UDs. However, when patients were stratified for disease phase and stem cell source, the difference in 3-year OS was not statistically different between MSDs and UDs (Table 2), except for patients with intermediate-risk disease receiving peripheral blood as a stem cell source (Table 2).
Patient selection
Several studies have addressed the issue of how to predict the outcome of SCT using clinical data. In addition to issues concerning the conditioning regimen or a specific disease, it may indeed be possible to anticipate transplantation complications and survival. Two predictive models are currently used: one is the so-called EBMT score33 and the other is the Co-morbidity score.34 The first is based on simple clinical characteristics, the second on more detailed comorbidities of the patients. The EBMT score is constructed on strong negative predictors of mortality, such as older patient age, advanced disease phase, long duration of disease, female donor in male recipient transplantations, and donor type. The EBMT score has been validated in many different settings, including second allogeneic transplantations. Currently, although the outcome of alternative donor transplantations is improving rapidly, the impact of patient age, disease status, and duration of disease remain significant variables so the EBMT score is still highly predictive. The comorbidity score34 has been confirmed by some studies and disproved by others. These predictive models can be very useful when discussing treatment strategies, a crucial moment when patient and physician come together to share prospects of the procedures and comparison with other treatment options.
Conclusions
In this review of studies comparing the outcome of UD and MSD transplantations comprising more than 30 000 patients, the average 3-year OS for MSDs is 46% and for UDs, 43%. The difference is currently small, but this is a rather general statement. Results can be quite different according to different diseases, and critical variables relating to the patient, the disease, and the transplantation need to be considered. In multivariate analysis, disease phase, patient age, GVHD prophylaxis, and stem cell source remain highly significant predictors of survival irrespective of donor type. In most studies, the donor type variable favors MSD, but in one unpublished study, young UDs did better than older siblings. As a general rule, the first choice of treatment remains MSD transplantation; however, refinement of HLA typing and improved understanding of natural killer alloreactivity on leukemic cells may provide the basis for the preference of UD transplantation in specific subsets of patients.
Acknowledgments
The author thanks Mohamad Mohty for providing data from the EBMT Acute Leukemia Working Party, Nicholas Kroeger for providing data from the EBMT Chronic Leukemia Working Party, Judith Marsh for providing data from the EBMT Severe aplastic anemia Working Party, Paul Veys for pediatric data, and Mary Horowitz for providing data and figures from the CIBMTR.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Andrea Bacigalupo, Divisione Ematologia e Trapianto di Midollo Osseo, IRCCS San Martino, Genova, Italy; Phone: 010-355469; Fax: 010-355583; e-mail: andrea.bacigalupo@hsanmartino.it.