Abstract
Acute venous thromboembolism poses significant problems in pregnancy, a time when objective diagnosis and prompt treatment are essential. Events can occur at any stage in pregnancy, but the period of greatest risk is in the weeks after delivery. Ultrasound venography remains the diagnostic technique of choice for deep venous thrombosis. For pulmonary thromboembolism, ventilation perfusion lung scan is usually preferred more than computerized tomography pulmonary angiography because of the lower maternal radiation dose and the lower prevalence of coexisting pulmonary problems. Low-molecular-weight heparin is the agent of choice for treatment of venous thromboembolism in pregnancy, and treatment should be provided for a minimum of 3 months and for at least 6 weeks after delivery. New anticoagulant agents such as dabigatran, rivaroxaban, or apixaban are not recommended for use in pregnancy.
Introduction
Pulmonary thromboembolism (PTE) remains a major cause of direct maternal mortality, with many deaths associated with a failure to obtain objective diagnoses (often because of unfounded concerns such as radiation exposure for the fetus) and lack of adequate treatment.1 The relative risk of antenatal venous thromboembolism (VTE) is approximately 5-fold higher in pregnant women than in nonpregnant women of the same age due to the changes in the coagulation and venous systems associated with pregnancy, but the absolute risk remains low at around 1 in 1000 pregnancies.2,3 Events are spread across the 3 trimesters, with more than 50% of events occurring in the first 20 weeks of pregnancy.4 The puerperium is the time of greatest risk, with estimates of relative risk of approximately 20-fold. Approximately 80% of events occur in the first 3 weeks after delivery,5 likely because of the addition of trauma to the pelvic vessels in the course of delivery causing endothelial damage. Compared with the nonpregnant woman, in whom distal deep venous thrombosis (DVT) is more common, most events in pregnancy are ileofemoral and left sided. The clinical diagnosis of DVT and PTE is particularly unreliable in pregnancy and after objective testing, only a minority of those with clinically suspected VTE will have the diagnosis confirmed. Acute VTE should be suspected during pregnancy when symptoms and signs consistent with possible VTE occur, such as unilateral and usually left-sided leg pain and swelling, lower abdominal pain, low-grade pyrexia, dyspnea, chest pain, and hemoptysis. The likelihood of VTE is higher when additional risk factors are present (Table 1) and when multiple risk factors combine the risk increase substantially, for example, when immobility is combined with a body mass index of ≥ 25 kg/m2. Although thrombophilia is a risk factor for VTE, performing a thrombophilia screen before commencing anticoagulant therapy is not recommended routinely because it will not influence the immediate management of acute VTE.
Risk factors and their odds ratios for risk of VTE in pregnancy5–8
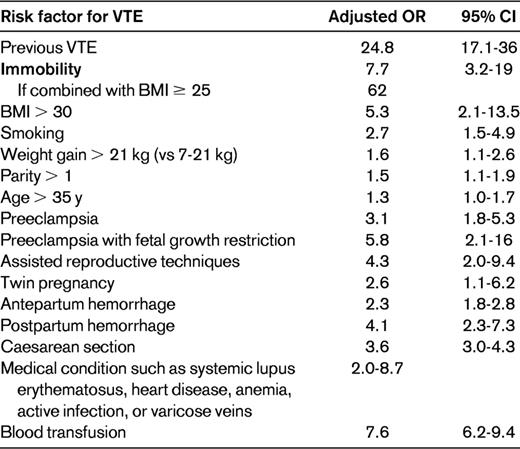
BMI indicates body mass index; CI, confidence interval; and OR, odds ratio.
Diagnosis of DVT in pregnancy
It is essential that objective diagnosis is sought in pregnant women with suspected VTE. If there is a delay in obtaining objective testing, anticoagulant therapy should be commenced until testing is available unless there are strong contraindications to its use.
Compression duplex ultrasound of the entire proximal venous system is considered the optimal first-line diagnostic test for DVT in pregnancy.9,10 If the initial ultrasound shows an abnormality in the popliteal or femoral veins, the diagnosis of proximal DVT is confirmed and therapeutic anticoagulation should be used. An apparently normal ultrasound examination in a patient with significant symptoms and signs or risk factors for VTE does not exclude a calf DVT, so serial ultrasound examinations should be repeated on days 3 and 7.10 If repeat testing is negative, anticoagulant treatment can be discontinued. When iliac vein thrombosis is suspected because the woman reports back pain and swelling of the entire limb, pulsed Doppler, magnetic resonance venography, or conventional contrast venography should be considered.9,10 Some practitioners use D-dimer measurements after the initial negative ultrasound scan and, when there is an elevated D-dimer, a repeat ultrasound is performed. However, D-dimer levels increase in normal pregnancy, increasing as gestation advances. D-dimer levels will be outside of the normal range at term and postpartum in most normal pregnancies. D-dimer levels also increase with complications such as preeclampsia and abruption, which are themselves associated with an increase in risk for VTE.9–11 Further, false-negative D-dimer results have been reported in cases of VTE in pregnancy.12 In view of these issues and because D-dimer assays have not been evaluated in prospective management studies, it is my practice to proceed directly to compression ultrasound venography in women with suspected DVT and to repeat this as required rather than use D-dimer measurements. A further consideration is the use of clinical prediction rules, which have value outside of pregnancy, but there are no large studies of such rules in pregnancy. In addition, because pregnant women frequently encounter leg swelling unrelated to thrombosis and are less likely to have medical comorbidities included as risk factors in these models, such rules may not be reliable in pregnancy10,11
Diagnosis of PTE
In the woman with a suspected PTE who is hemodynamically stable, a chest X-ray is valuable to identify other pulmonary diseases such as pneumonia or pneumothorax. Pregnant women in general have low rates of preexisting pulmonary disease and, in more than 50% of cases, the chest X-ray will be normal. Nonspecific features of PTE on chest X-ray include atelectasis, effusion, focal opacities, regional oligemia, and pulmonary edema. The radiation dose to the fetus from a chest X-ray performed at any stage of pregnancy is negligible and this test should not be withheld from a pregnant woman with a potentially fatal condition. If the chest X-ray is abnormal with a high clinical suspicion of PTE, then ventilation perfusion scanning, the preferred objective test for suspected PTE in pregnancy, is unreliable and computed tomography pulmonary angiography (CTPA) should be performed.9 When the chest X-ray is normal, I then proceed to Doppler ultrasound venography because a diagnosis of DVT may confirm PTE indirectly and anticoagulant therapy is the same for both conditions. Therefore, further pulmonary investigation may not be necessary, thus avoiding the radiation doses, particularly those associated with CTPA, for the mother and fetus. It is important to consider the issue of radiation exposure in the context of diagnosis of PTE. Concerns over radiation exposure for the fetus are often cited as reasons for avoiding radiation-based investigations in pregnancy. However, the tests used most commonly are not associated with high levels of fetal exposure. In addition, the context of a potentially fatal disorder for the mother and the fetus if the event is antenatal should be considered. CTPA is associated with less radiation exposure to the fetus than ventilation/perfusion (V/Q) lung scans in all trimesters of pregnancy. It has been estimated that the risk of fatal cancer up to the age of 15 years is < 1 in 1 000 000 after in utero exposure to CTPA and 1 in 280 000 after a perfusion scan.9 Perhaps of more concern is that although CTPA is associated with a lower dose of radiation for the fetus than a V/Q scan, it exposes the mother to a relatively high radiation dose: as much as 20 mGy to the thorax and in particular breast tissue. It has been calculated that this is associated with a significant increase in the lifetime risk of breast cancer, because breast tissue is especially sensitive to radiation exposure during pregnancy. Pulmonary angiography carries the highest radiation exposure (at least 0.5 mSv to the fetus and 5-30 mSv to the mother).
To summarize, the main techniques for objective diagnosis of PTE are V/Q lung scans or CTPA. The choice may be restricted by local availability and guidelines. Where available, V/Q scans are generally preferred because of the lower radiation dose to the mother and the low incidence of comorbid pulmonary problems that often reduce the value of such scans in the nonpregnant patient.9 Further, during pregnancy, and especially if the chest X-ray is normal, the ventilation component can often be omitted, thereby minimizing the radiation dose for the fetus. In the nonpregnant woman, CTPA is usually the first-line investigation for nonmassive PTE due to better sensitivity and specificity than the V/Q lung scan. It can also identify other pathology such as aortic dissection, but, as noted, the radiation dose is an important concern when only approximately 5% of such investigations will have a positive result. For these reasons, I prefer V/Q lung scan over CTPA for the first-line investigation of PTE in pregnancy because: (1) it has a high negative predictive value, (2) most pregnant women will not have comorbid pulmonary pathology, and (3) it has a substantially lower radiation dose to the breast tissue.
Management of VTE in pregnancy
Initial assessment
In the initial assessment of the pregnant patient before commencing therapeutic anticoagulation for VTE, complete blood count, coagulation screen, urea, electrolytes, and liver function tests should be performed to exclude renal or hepatic dysfunction, which are risk factors for anticoagulant therapy. Thrombophilia screen should not be performed because many factors are disturbed by both pregnancy and the presence of thrombus and because the results will not alter the acute management of VTE.
Anticoagulants in pregnancy
Vitamin K antagonists cross the placenta. They are associated with warfarin embryopathy with first trimester exposure, an increased risk of pregnancy loss, CNS abnormalities with later pregnancy exposure, and a risk of fetal and neonatal bleeding around the time of delivery due to fetal anticoagulation. However, coumarin can be used postpartum if required, because there is no significant excretion in breast milk. There are limited data on the new anticoagulant therapies in pregnancy. Although there are some limited data on fondaparinux in pregnant women, which are reassuring with regard to adverse outcomes, because of the substantially greater experience with low-molecular-weight heparin (LMWH) and its established safety, it is recommended rather than fondaparinux. The latter should be restricted to those patients with severe adverse reactions to heparin, such as those with heparin-induced thrombocytopenia (HIT), who cannot be given danaparoid.11 Further, because there are no significant data supporting the use of new oral direct thrombin inhibitors (eg, dabigatran) or the new anti-Xa inhibitors (eg, rivaroxaban and apixaban),11 and because these relatively small molecules may cross the placenta with consequent adverse fetal effects, these should be avoided in pregnancy at present.11 Therefore, heparins remain the agents of choice for the treatment of VTE in pregnancy.9,11
LMWH has been largely replaced with unfractionated heparin (UFH) for the immediate management of VTE in pregnancy. Neither UFH nor LMWH crosses the placenta or is present in breast milk in appreciable amounts. There are now substantial data from randomized controlled trials in nonpregnant patients confirming that LMWH is more effective than vitamin K antagonists in preventing recurrent VTE and postthrombotic syndrome without increasing the risk of serious bleeding events.11,13 Further, LMWH is more effective, has a lower risk of bleeding, and is associated with lower mortality than UFH for the initial treatment of DVT in nonpregnant patients.9,11,14 In addition, a meta-analysis of randomized controlled trials has shown equivalent efficacy of LMWH and UFH in the initial treatment of PTE.10,14 Whereas the data on efficacy are extrapolated from those in nonpregnant women, direct data on safety are available for pregnant women. A systematic review of LMWH in pregnant women has confirmed its safety and also describes efficacy consistent with that in nonpregnant women in the management of acute VTE.15,16 Compared with UFH, LMWH is associated with a substantially lower risk of HIT, hemorrhage, and osteoporosis.10,15,16
Acute VTE
In the nonpregnant woman, acute VTE is usually managed with LMWH in a once-daily dose. However, in pregnant women, a twice-daily regimen is often recommended9 because of alterations in the pharmacokinetics of LMWH, which is cleared by the kidney. There are growing data, mostly with tinzaparin, that once-daily dosing may be satisfactory (tinzaparin 175 units/kg) and may be appropriate in the treatment of VTE in pregnancy.16,17 Because there is greater experience with twice-daily dosing and because of the possibility of reduced anticoagulant activity toward the end of the 24-hour period due to increased renal clearance, I prefer a twice-daily dose with enoxaparin (1 mg/kg twice daily) or dalteparin (100 units/kg twice daily) in the initial treatment of acute VTE until the situation is stable. At that time, I convert to a once-daily regime of 1.5 mg/kg of enoxaparin or 10 000-18 000 units of dalteparin once daily depending on body weight. Because the patient will remain on LMWH for some time, she should be taught to self-administer the LMWH by subcutaneous injection. Once the situation is stable and she is confident with self-administration, out-patient management is satisfactory until delivery.
DVT
It is important to remember that other techniques are also of value in the management of acute DVT in pregnant women. Leg elevation should be used and graduated elastic compression stockings fitted to reduce edema. Thereafter, the woman should be encouraged to mobilize while wearing compression stockings, as is also recommended in nonpregnant patients13 to reduce pain and swelling. Studies in nonpregnant women have shown that early mobilization along with compression therapy does not increase the likelihood of developing PTE.18,19 This approach may also help to prevent the development of postthrombotic syndrome. There are some trial data in nonpregnant women to suggest that compression stockings started within 2 weeks of DVT and continued for 2 years reduces the likelihood of postthrombotic syndrome by approximately 50% but does not affect the frequency of recurrent VTE.13 Although there are no data demonstrating an impact from compression stockings on clinical outcomes in gestational DVT, the effect of these stockings on the venous system in postpartum women has been studied and are associated with reduced diameter of the common femoral vein and increased blood flow velocity.20
Treatment of DVT by thrombolysis can reduce postthrombotic syndrome, but at the expense of increased bleeding.13 In the nonpregnant woman, the current American College of Chest Physicians recommendation is to prefer anticoagulation over systemic thrombolysis, reserving consideration of the latter only for patients with all of the following criteria: iliofemoral DVT, symptoms for < 14 days, good functional status, life expectancy of ≥ 1 year, and low risk of bleeding.13 Catheter-directed thrombolysis is considered the preferable approach. Because of the uncertainty regarding the balance of risks and concerns relating to major bleeding, during pregnancy, anticoagulant therapy alone appears preferable to systemic thrombolysis unless there is massive occlusive ileofemoral DVT threatening leg viability through venous gangrene.
Life-threatening PTE
The pregnant woman with a massive, life-threatening PTE is a serious obstetric and medical emergency. This may be defined as a pulmonary embolus associated with hemodynamic compromise (systolic blood pressure < 90 mmHg or a decrease in systolic blood pressure of ≥ 40 mmHg from baseline for a period > 15 minutes) not otherwise explained by hypovolemia, sepsis, or new arrhythmia. The collapsed, shocked pregnant woman requires rapid assessment by a multidisciplinary resuscitation team of experienced clinicians, including senior obstetricians, physicians, and radiologists. They will quickly assess the situation and decide the treatment on an individual basis taking into account the available resources and expertise. The options include: IV UFH, thrombolytic therapy, catheter-assisted thrombus removal, and surgical embolectomy. In the initial response, oxygen should be administered and circulatory support provided with IV fluids and inotropic agents as required. Intravenous UFH is the traditional method of heparin administration in acute VTE and remains the preferred treatment in massive PTE because of its rapid effect and our extensive experience of its use in this situation. This is a situation in which there is a strong case for considering systemic thrombolytic therapy because anticoagulant therapy will not reduce the obstruction of the pulmonary circulation. An infusion of UFH can be given after thrombolytic therapy. There is growing evidence11,21–23 on the use of thrombolytic agents in pregnancy. Streptokinase, and probably other thrombolytic agents as well, do not cross the placenta. No maternal deaths associated with thrombolytic therapy have been reported, and the maternal bleeding complication rate is approximately 6%, which is consistent with that in nonpregnant patients receiving thrombolytic therapy. Most bleeding events occur around the catheter and puncture sites and, in pregnant women, in the genital tract. If the patient is not suitable for thrombolysis or is moribund, cardiothoracic surgeons should be consulted urgently for consideration of emergency thoracotomy.
IVC filters
It is my experience that inferior vena cava (IVC) filters are rarely necessary and in general should be restricted to women with proven VTE and continuing PTE despite adequate anticoagulation or those in whom anticoagulation cannot be used. Temporary IVC filters can be considered in the perinatal period for large iliac DVT, but their value is uncertain. Each case requires individual assessment because there are hazards from filter placement, including filter migration, which occurs in > 20% of patients; filter fracture, which occurs in approximately 5% of patients; and IVC perforation, which occurs in up to 5% of patients. In the nonpregnant patient, filters reduce PTE, increase DVT, but have no change in overall frequency of VTE.9,11,13 Finally, temporary filters remain in situ in a large proportion of patients. When a woman enters pregnancy with a filter in situ, her anticoagulation therapy must be reviewed. If she is on coumarin therapy, then this should be switched to intermediate or treatment dose LMWH by 6 weeks of gestation to avoid the risk of warfarin embryopathy. Outside of pregnancy, patients with filters in place increasingly do not remain on anticoagulation indefinitely; for example, they may be taken off of it when the risk factor for thrombosis resolves. This is done because of the relative risk of bleeding when a patient is on long-term warfarin relative to the risk of thrombosis. However, filters increase the risk of DVT without eliminating the risk of PTE. Further, LMWH does not carry the same risk of serious bleeding problems as long-term coumarin therapy. Pregnancy increases the risk of thrombosis substantially. Therefore, in women with a filter in place who become pregnant, anticoagulation should be continued or restarted (with LMWH) as soon as pregnancy is diagnosed.
Monitoring of LMWH therapy
Although useful in monitoring UFH, the activated partial thromboplastin time is not changed significantly in patients on LMWH and therefore cannot be used to assess response to therapy. Monitoring anti-Xa levels for women on LMWH is no longer recommended9 and is difficult to justify in pregnancy due to the satisfactory results with weight-based dosing. In addition, anti-Xa monitoring is not useful in predicting either recurrent thrombosis or bleeding risk, at least in part because of variability in the assay.9,11,24 There may be a case for monitoring levels for women at extremes of body weight (< 50 kg and > 90 kg) or those with other complicating factors such as renal disease, severe thrombophilia, and recurrent VTE. If LMWH therapy requires monitoring, the aim is to achieve a peak anti-Xa activity of 0.5-1.2 μ/mL 3 hours postinjection for women with VTE. Routine platelet count monitoring for evidence of HIT is not required in pregnant patients who have received only LMWH. However, if the patient is receiving LMWH after first receiving UFH or if she has received UFH in the past, making HIT more likely, the platelet count should be monitored every other day from days 4-14 or until LMWH is stopped, whichever occurs first.25
Duration and intensity of maintenance treatment of VTE
Women with antenatal VTE can be managed with subcutaneous LMWH for the remainder of the pregnancy. It is uncertain whether dose adjustment is required in pregnancy in relation to pregnancy-associated weight gain. It is my practice to continue with the initial dose regimen throughout pregnancy for the majority of patients despite the pregnancy-associated weight gain, because LMWH does not cross the placenta and therefore the weight of the feto-placental unit is not relevant.9,11 It is not yet established whether the initial dose of LMWH can be reduced to an intermediate dose (ie, 75% of treatment dose) after an initial period of several weeks of therapeutic anticoagulation. However, this practice has been used successfully outside of pregnancy in patients with contraindications to coumarin therapy and in patients with underlying malignancies.11 Although there have been no studies comparing these 2 types of dosing strategies in pregnant women directly, this type of modified dosing regimen may be useful in pregnant women at increased risk of bleeding or osteoporosis. For pregnant women with acute VTE, it is recommended that anticoagulants should be continued for at least 6 weeks postpartum and for a minimum total duration of therapy of 3 months.9,11
Considerations for delivery in the women on anticoagulant treatment for VTE
For women on therapeutic anticoagulation, a planned delivery, either through the induction of labor or by elective caesarean section, may allow optimal timing of events and minimizes the risks of an unplanned delivery on full anticoagulation. Each patient requires individual management depending on the thrombotic and bleeding risks. However, in general and in the absence of bleeding complications arising from delivery, LMWH should be reduced to a once-daily thromboprophylactic dose on the day before induction of labor or caesarean section. A further thromboprophylactic dose of LMWH (enoxaparin 40 mg; dalteparin 5000 units; tinzaparin 75 units/kg) should be given by 3 hours postoperatively or > 4 hours after removal of the epidural catheter if appropriate, and the treatment dose recommenced approximately 12 hours later. There is an increased risk of wound hematoma after caesarean section with both UFH and LMWH of approximately 2%. For this reason, wound drains should be considered at the time of caesarean section and the skin incision should ideally be closed with staples or interrupted sutures to allow easy drainage of hematoma. When a woman presents in labor on therapeutic LMWH, neuraxial anesthetic techniques should usually be avoided for at least 24 hours after the last dose of LMWH.
A problem that causes concern frequently is the situation in which thrombosis occurs close to term with a consequently high risk of spontaneous labor. Consideration should be given to the use of UFH in this case because it can be relatively easily reversed using protamine sulfate and has a short duration of action. If spontaneous labor occurs in women receiving therapeutic doses of subcutaneous UFH, assessment of the anticoagulant effect by the activated partial thromboplastin time is required. Subcutaneous UFH should be discontinued 12 hours before and IV UFH stopped 6 hours before the induction of labor or regional anesthesia.
Postnatal anticoagulation
Both heparin and warfarin are satisfactory for use postpartum (both heparins and coumarin are satisfactory for breastfeeding mothers). In my experience, most women prefer to use LMWH, which can be used with once-daily dosing postpartum, because they have become accustomed to its administration and they appreciate the convenience of not having to go to the clinic for monitoring of coumarin therapy when caring for a new baby. Before discontinuing treatment, the ongoing risk of thrombosis should be assessed, including a review of personal and family history of VTE. Thrombophilia screening should be discussed and arranged if required. It has been reported recently26 that low-dose aspirin can prevent a recurrence of VTE in nonpregnant patients after completion of 6-18 months of anticoagulant therapy and is associated with a low risk of bleeding. These data do not suggest that low-dose aspirin can replace therapeutic anticoagulation for VTE, but do suggest that when prolonged therapy is considered to be of benefit after the completion of a full course of therapeutic anticoagulation, it may be valuable. These data also do not suggest that low-dose aspirin can replace LMWH in pregnant women with a previous VTE. In view of the risk of VTE and its importance for maternal mortality and morbidity, LMWH, for which there is now a substantial evidence base of efficacy and safety, remains the leading therapeutic option for the secondary prevention of VTE in pregnancy.
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Leo Pharma and Sanofi. Off-label drug use: LMWH in pregnancy.
Correspondence
Ian A. Greer, Faculty of Health and Life Sciences, University of Liverpool, Foundation Building, Liverpool L69 7ZX, United Kingdom; Phone: 44-151-795-0422; Fax: 44-151-795-5256; e-mail: ian.greer@liverpool.ac.uk.
