Abstract
Imatinib has been the preferred initial therapy for newly diagnosed chronic myeloid leukemia patients for the past 10 years. Recently, other, possibly better, tyrosine kinase inhibitors have been licensed for first-line use based on the early results of 2 large, randomized clinical trials. The pros and cons of the various alternatives to imatinib are analyzed herein, and I try to answer the question of are we ready to abandon imatinib and, if yes, then what treatment should a patient diagnosed today receive.
Introduction
Ten years ago, chronic myeloid leukemia (CML) therapy was relatively straightforward: all patients received imatinib as first-line treatment and for those who failed imatinib, the only available proven alternative was allogeneic stem cell transplantation. Subsequently, newer, allegedly more potent, tyrosine kinase inhibitors (TKIs) displaced allogeneic stem cell transplantation as the second-line (and eventually as the third-line therapy) of choice. In recent years, some of these drugs, namely nilotinib and dasatinib, have been licensed for first-line use on the basis of 2 randomized studies showing a slightly higher proportion of patients achieving early complete cytogenetic responses (CCyRs) on nilotinib1 and dasatinib2 compared with imatinib-treated controls. Today, perplexed practitioners struggle in the clinic to choose the most appropriate initial treatment for their CML patients. The decision-making process is not simple, because there is almost no solid evidence that can help select one particular drug in preference to another. Herein, I will try to provide some aid to physicians facing the challenging question: what should I give to this new patient?
Setting the scene: what can be achieved with imatinib?
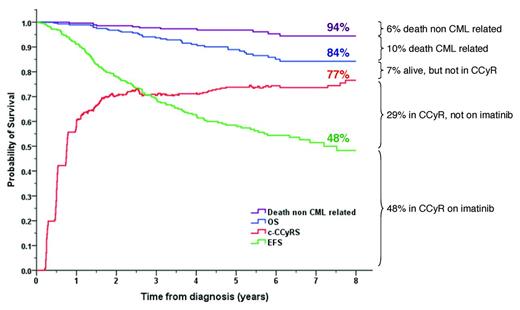
We have more than 12 years of experience using imatinib as first-line therapy for CML. The drug is safe, it has been taken by hundreds of thousands of patients, and there is a good understanding of the side-effect profile. Approximately 70% of patients who receive imatinib as first-line therapy will achieve CCyR by 12 months, and 80% will have done so by 5 years.3,4 In a series of 282 patients treated at Hammersmith Hospital in London, the 8-year probability of overall survival (OS) on an “intention-to-treat” basis was 84%, the probability of being alive in CCyR was 77%, but the probability of imatinib failure-free survival was only 50%. This shows that although the majority of patients who commence imatinib therapy will fare well, a significant proportion will need a change of therapy due to unsatisfactory responses or side effects (Figure 1).5
Outcome of 282 patients treated with imatinib as first-line therapy at the Hammersmith Hospital. c-CCyRS (current CCyR survival) was defined as the probability of being alive and in CCyR at a given time point. The c-CCyRS is the analog of “current leukemia free-survival” and takes into account the fact that a patient who relapses on one drug may reachieve a CCyR on a second-line treatment. An event was defined as loss of a CCyR or CHR, progression to advanced phase, death, or imatinib discontinuation. (Figure is based on Marin et al.5 )
Outcome of 282 patients treated with imatinib as first-line therapy at the Hammersmith Hospital. c-CCyRS (current CCyR survival) was defined as the probability of being alive and in CCyR at a given time point. The c-CCyRS is the analog of “current leukemia free-survival” and takes into account the fact that a patient who relapses on one drug may reachieve a CCyR on a second-line treatment. An event was defined as loss of a CCyR or CHR, progression to advanced phase, death, or imatinib discontinuation. (Figure is based on Marin et al.5 )
Imatinib, nilotinib, dasatinib, and bosutinib: results, advantages, and disadvantages
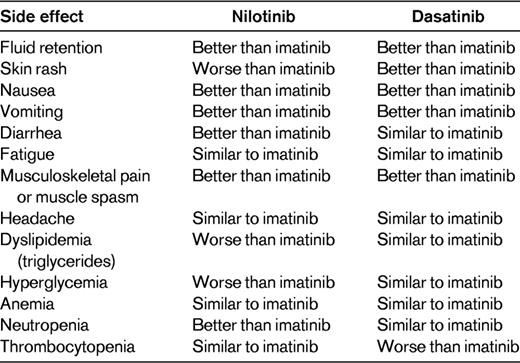
Side effects differ from drug to drug, but it is possible to define a drug class side effect profile, namely: myelosuppression (the main side effect), tiredness, fluid retention, gastrointestinal disturbances, hepatotoxicity, myalgia, arthralgia, skin rash, QTc interval prolongation, hypocalcaemia, hypophosphatemia, and increased levels of amylase and lipase. All 4 drugs can cause these side effects, but the relative frequency of the individual side effect differs for each drug; for example, facial edema is very frequent on imatinib and rare on dasatinib. Table 1 shows the main differences in the side-effect profile of the 3 licensed drugs.
Imatinib
Imatinib is the first drug of its class. It is given once daily, normally with food (to prevent nausea). Nausea, myalgia, arthralgia, and fluid retention are seen far more frequently on imatinib than on the other drugs (Table 1). Nausea and fluid retention can be easily managed, but arthralgia and myalgia may persist in a minority of patients despite optimal support.
Advantages.
Imatinib is the only drug with which we have long-term (>10 years) experience. Its side-effect profile is well known. In most countries, it is the least-expensive drug, and it is likely to become even less expensive because generic preparations will soon be available world-wide.
Disadvantages.
Imatinib probably induces a higher proportion of insidious low-grade side effects (eg, muscle cramps) than nilotinib and dasatinib. The proportion of patients who achieve CCyR by 1 year is lower than with nilotinib or dasatinib.
Nilotinib
Nilotinib is a BCR-ABL1 inhibitor that was rationally designed to be more potent and selective than imatinib. Until recently, it had been used mostly and quite successfully as a second-line agent, and it is now licensed for first-line use at a dose of 300 mg twice a day. Almost all of the information regarding nilotinib comes from the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) trial, in which 846 patients were randomly allocated to receive imatinib 400 mg once per day, nilotinib 300 mg twice per day, or nilotinib 400 mg twice per day.1,6 For the purposes of this analysis, only the dose of nilotinib of 300 mg twice a day will be considered. Table 2 summarizes the responses obtained in the study. Perhaps the most important result is that by 1 year, the cumulative incidence of CCyR was 15% higher in the patients receiving nilotinib than in imatinib controls.6 Furthermore, patients receiving nilotinib had a much higher probability of achieving a major molecular response (MMR or MR3). However, this superior rate of responses has not yet translated into improvements in progression-free survival (PFS) and OS (Table 2). Nilotinib is well tolerated and causes less nausea, myalgia, arthralgia, and fluid retention than imatinib (Table 1). However, it produces skin rashes or pruritus in the majority of patients, but in most cases these can be easily controlled with antihistamines. Nilotinib induces hyperglycemia in approximately 40% of patients and also causes increases in the triglyceride levels. It is the most hepatotoxic of the 4 drugs, although this is normally limited to mild increases in transaminases that do not require action. A severe toxic hepatitis occurs rarely. Similarly, nilotinib causes an increase in the bilirubin level in the majority of patients but, again, this seldom necessitates any modification of therapy.
Results of the randomized trials comparing nilotinib 300 mg twice per day versus imatinib 400 mg once per day (ENESTnd), dasatinib 100 mg once per day versus imatinib 400 mg once per day (DASISION), and bosutinib 500 mg once per day versus imatinib 400 mg once per day (BELA)
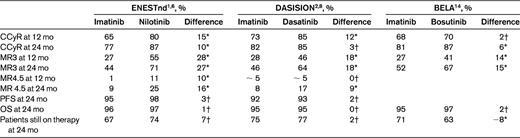
The rates of response (CCyR, MR3, and MR4.5) are given as cumulative incidences. MR3 (MMR) is defined as a 3-log reduction in transcript levels or 0.1% on the international scale. PFS and OS are expressed as 2-year probabilities. Patients still on therapy at 24 mos are expressed as proportions. The columns with the heading “difference” indicate the difference in outcome between the second-generation TKI and imatinib.
*Statistically significant difference.
†Statistically nonsignificant difference.
Advantages.
A higher proportion of patients achieve CCyR by 1 year with nilotinib. It is well tolerated, even more so than imatinib if one excludes the skin rash.
Disadvantages.
Nilotinib has a very complicated posology; it is given every 12 hours and patients have to fast for 2 hours before taking it and for 1 hour afterward. This is possibly the main limitation of the drug. Skin toxicity, hyperglycemia, and increases in triglyceride levels also may occur. The side-effect profile is not yet fully understood; for example, nilotinib has been associated with progressive peripheral arterial occlusive disease,7 although the incidence of this complication is not yet clear. In the majority of countries, nilotinib is significantly more expensive than imatinib.
Dasatinib
Dasatinib is a multitarget kinase inhibitor that is more than 300 times more potent than imatinib in inhibiting the BCR-ABL1 oncoprotein in vitro.2,8 As with nilotinib, most of the experience to date has been with using dasatinib in patients who have failed imatinib. Dasatinib is the only second-generation TKI licensed for use in advanced-phase patients. Again, most of the information on first-line use comes from a single multicenter, industry-sponsored study, the Dasatinib versus Imatinib Study in Treatment-Naive CML Patients (DASISION) trial, in which 519 CML patients were randomized to receive either dasatinib 100 mg once per day or imatinib 400 mg once per day as first-line therapy.2,8 Table 2 summarizes the results of the study. Briefly, dasatinib induces a higher proportion of CCyR at 12 months (12 percentage points higher than imatinib). However, this difference had disappeared by 24 months. Like nilotinib, dasatinib induces MRs in a considerably higher proportion of patients than imatinib controls.8 Dasatinib is well tolerated because it has a lower incidence of insidious low-grade side effects than imatinib (Table 1). Pleural effusions are the main complication of dasatinib therapy, but the reported incidence varies in different series. Most studies report an incidence of below 25%, although cumulative incidences as high as 54% have been reported.8–12 In our institution, in 140 chronic-phase patients treated with dasatinib after imatinib failure, the 5-year cumulative incidence is 32.5%. Because pleural effusions can occur, some after starting treatment, the proportion of patients who will eventually be affected is not known. Pleural effusions are easy to manage. Diagnostic thoracocentesis is not usually required, and in practice, the effusion nearly always resolves on discontinuing the drug without the need for any invasive procedure. When a pleural effusion is suspected, our practice is to confirm the diagnosis with a chest X-ray, interrupt the dasatinib, and treat the patient with 0.5 mg/kg of prednisolone for 1 or 2 weeks. Pleural effusions may recur despite dose reduction.
Advantages.
Dasatinib is licensed for advanced-phase disease. A higher proportion of patients achieve CCyR by 1 year than with imatinib. It is well tolerated, even more so than imatinib if one excludes the pleural effusions. It is given once daily and can be taken with food or fasting (unlike imatinib and nilotinib).
Disadvantages.
There is a high incidence of pleural effusions associated with dasatinib. The side-effect profile is not yet fully understood, and dasatinib has been associated with pulmonary arterial hypertension. The incidence of this complication is not clearly established (but is low), and it seems to be reversible on discontinuation of the drug.13 As is the case with nilotinib, in most countries, dasatinib is significantly more expensive than imatinib.
Bosutinib
Bosutinib is a dual SRC and ABL TKI that is not currently licensed for first- or second-line use. A large phase 3 clinical trial, Bosutinib Efficacy and safety in chronic myeloid LeukemiA (BELA), has been completed recently, and the drug may soon be licensed for first-line use. In the BELA study, 502 patients were randomized to receive 500 mg once per day of bosutinib or 400 mg once per day of imatinib.14 The 1-year cumulative incidence of CCyR was similar with both drugs, but bosutinib induced a higher proportion of MRs (Table 2). The drug is well tolerated. Its main side effect is diarrhea, which can easily be managed symptomatically.
Advantages.
Bosutinib has easy posology and is perhaps less toxic than imatinib.
Disadvantages.
Bosutinib is associated with frequent diarrhea.
The objective of treatment: CCyR and MR
The achievement of CCyR is the major objective of therapy because it is associated with prolonged survival.3,4,15–18 In patients who achieve CCyR, the BCR-ABL1 transcript levels may be monitored to assess the quantity of residual leukemia, and results are often expressed as the log10 reduction from a standardized value for untreated patients, or more recently using the international scale where 100% is the starting point. It is generally accepted that CCyR corresponds to an approximate 2-log reduction in transcript levels or 1% on the international scale.19 MMR (or perhaps better, MR3) is usually defined as a 3-log reduction in transcript levels or 0.1% on the international scale. Similarly, MR4 is defined as a 4-log reduction (0.01% on the international scale) and MR4.5 is defined as a BCR-ABL1 ratio lower than 0.0032% on the international scale provided copy numbers for the control gene are at least 32 000 (ie, a 4.5-log reduction). The term complete MR (CMR) means that the transcripts are no longer detectable at a given sensitivity level (commonly 4, 4.5, or 5 logs), but this is in no way an indication that leukemia has been eliminated from the patient.
Both nilotinib and dasatinib have been licensed for first-line use on the basis of a modest improvement in the proportion of patients obtaining CCyR, but both drugs are clearly superior to imatinib in rapidly inducing MR3, MR4, and MR4.5 (Table 2).1,2 Should we prefer dasatinib or nilotinib over imatinib for this reason? Possibly not. Whereas there is overwhelming evidence that the achievement of CCyR prolongs life, there is no proof that the achievement of MR3, MR4, MR4.5, or CMR by patients who are already in CCyR has any added benefit in terms of OS or progression to advanced-phase disease. In fact, there is a considerable body of evidence suggesting that there is no additional benefit from achieving these molecular targets in terms of OS or PFS.3,5,20,21 For this reason, no specific TKI should be preferred over the others solely on the basis that it induces a higher proportion of MRs.
Which is the best drug?
The best drug is not necessarily the most efficacious. It is also important to consider other factors such as toxicity, in particular when, as in this case, the differences in efficacy between them are not dramatic. One way to estimate the overall “goodness” of a drug is to examine how many patients are still taking the drug at a given time point, because this provides a combined estimate of efficacy, toxicity, and other factors that may affect therapy failure.4 Interestingly, both the DASISION and the ENESTnd trials reveal that the proportion of patients who are still taking the drug allocated at randomization 2 years down the line is very similar: imatinib-dasatinib, 75% versus 77%, and imatinib-nilotinib, 67% versus 74%.6,8 The fact that both trials showed that the number of patients still taking the initial therapy 2 years later was practically identical in both arms indicates that the superior efficacy of nilotinib and dasatinib is being offset by other factors, most likely side effects, that warrant permanent discontinuation of the drug. The pros and cons of the different TKIs are balanced in a way that makes it very difficult to decide which is the best for front-line therapy for CML; for example, is having to fast for 3 hours twice a day a good price to pay for a slightly higher 1-year CCyR rate? Which is worse, a 20% risk of having a relatively benign pleural effusion or having to fast every time one takes the drug? The answer depends on the specific weighting that we give to each of the possible complications or inconveniences of the various drugs, and that has to be established in discussion with the patient.
Low-grade side effects
TKIs are generally well tolerated. Serious side effects such as cardiotoxicity or hepatitis are rare and usually resolve on discontinuing the drug without causing permanent damage. Deaths due to toxicity of TKIs are exceedingly rare. Conversely, low-grade side effects are very common. Whereas it is true that some patients feel completely normal on TKI therapy, a substantial proportion of patients suffer 1 or other low-grade side effect, most commonly asthenia, skin rashes, muscle cramps, or hair loss. The 3 available TKIs have different relative frequencies of these various side effects (Table 1), but no one drug seems to be clearly better than the others regarding the proportion of patients who are completely free of side effects. Therefore, when we choose one drug over another, we are in effect choosing which side effects the patient is more likely to suffer (eg, skin rash vs fluid retention) rather than whether the patients will or will not be totally free of side effects. The importance of low-grade side effects should not be underestimated, because they may have a large impact on the quality of life of patients (for example, suffering even grade I asthenia for 15 years). Furthermore, the presence of low-grade side effects is the main cause for poor compliance to medication in the longer term, and poor compliance has been shown to be the major contributor to relapse in patients in stable CCyR.22,23 Because it is impossible to know beforehand with which drug the patient will feel more comfortable, a reasonable approach is to begin therapy with 1 of the 3 TKIs and then, if the patient suffers from low-grade side effects that cannot be satisfactorily relieved with optimal management, consider changing to an alternative. One should be careful not to make unnecessary or frivolous changes in medication. Changes in medication on these grounds should also be avoided during the first months of therapy, because it may then prove difficult to classify the patient as responder or nonresponder to TKI therapy.
Alternative initial therapies
High-dose imatinib
From the time that imatinib entered clinical practice, researchers were interested in exploring the use of higher doses (600 or 800 mg once per day). Results of a single-arm phase 2 trial showed higher rates of CCyR and MRs.24 However, several randomized trials have failed to show any long-term benefit of higher doses of imatinib on PFS and OS (although the proportion of MRs was higher).25–27
Imatinib plus IFN-α
IFN-α is an active agent against CML. It induces CCyR in 5%-20% of patients, which is associated with a survival greater than 10 years.15 IFN-α was the standard first-line therapy until the advent of imatinib. Logically, there was great interest in the possibility of combining both agents to improve the results of imatinib alone. However, the results have been somewhat disappointing, because large randomized clinical trials have either found no benefit with the combination28 or a benefit limited to higher rates of MRs but with no impact on the rates of CCyR, PFS, or OS.27,29 Today, the combination of IFN-α with TKIs is not used outside clinical trials.
I am familiar with prescribing imatinib: can I still use it as my preferred first-line agent?
The major consideration in answering this question is whether dasatinib or nilotinib reduces the number of patients who progress to the advanced phase in the course of the disease. In the 2 randomized clinical trials mentioned above, both dasatinib and nilotinib showed a trend toward better PFS than imatinib (in fact, nilotinib 400 mg twice a day showed a significantly better PFS compared with imatinib, but this was not the dose taken forward in the application for the first-line use license). It is possible that longer follow-up will eventually show a clear advantage in PFS or OS for one of these agents. Having said that, it is not clear how we can incorporate this information into our clinical practice, because the way that patients were managed in the trials is different from what we would accept as appropriate today. For example, in the ENESTnd trial, the criteria used to define a patient as having lack of response to initial therapy and thus allowing a change of drug were: (1) no complete hematologic response by 6 months, (2) no cytogenetic response by 6 months (>95% Philadelphia chromosome positive), (3) no major cytogenetic response by 12 months (>35% Philadelphia chromosome positive), and (4) no CCyR by 18 months, and this only after having had the dose of imatinib or nilotinib escalated. These criteria used to define resistance are different from what today would be considered as best practice, namely to assess the patient at 3 months and to change therapy if necessary. This assessment can be done by measuring the BCR-ABL1 transcripts5,30,31 or by conventional BM cytogenetics.32 It is important to stress that the molecular assessment should be based on the use of an appropriately standardized methodology.33,34 We have shown that patients who at 3 months have a BCR-ABL1/ABL1 transcript ratio greater than approximately 10% (equivalent to MCyR) have a significantly worse PFS and OS than those with lower transcripts.5 The same cutoff can be used for patients treated with a second-generation TKI, but more precise estimates can be obtained by lowering the cutoff to 2% (equivalent to CCyR).30 A similar set of criteria has been proposed by investigators at the MD Anderson Cancer Center, namely the achievement at 3 months of MCyR for imatinib-treated patients or CCyR for nilotinib- or dasatinib-treated patients.18,32 Using early intervention strategies, it is likely that we will be able to improve the outcome of patients who receive imatinib first-line and thus close the small (and not yet significant) gap in PFS between imatinib and nilotinib or dasatinib. Whether patients treated with dasatinib and nilotinib first-line will benefit from an early intervention strategy is not clear. There are no data on response to imatinib, dasatinib, or nilotinib as second-line therapy in patients who receive nilotinib or dasatinib as first-line therapy, but I think there are reasons for concern, because we know that patients with primary cytogenetic resistance who have failed imatinib and also second-line dasatinib or nilotinib have a very low probability of responding to a third-line agent.35 To summarize, nilotinib and dasatinib have not yet shown significant superiority in terms of PFS and OS, although there is a trend in that direction, in particular by reducing early progression. This trend is likely to become significant with longer follow-up times. However, the possible superiority of the newer drugs is based on comparing the new agents to imatinib used in an old-fashioned way. Therefore, physicians who feel comfortable using imatinib do not need to abandon this treatment provided that the patient is assessed for response early after starting therapy (ie, at 3 months) and that patients who fail to achieve the required degree of response (ie, those with BCR-ABL1/ABL1 ratios > 10% or no MCyR) are then treated with a second-line agent.
Situations in which I would choose a particular drug for a patient and why
The 3 available TKIs have different side-effect profiles, but the side-effect profile will only very occasionally be the determining factor in our choice of TKI for a given patient. For example, nilotinib can increase the glucose level in a significant proportion of patients, and one could make a case for treating a diabetic patient with other agents, but in practice it has been shown that nilotinib can be used safely in diabetic patients merely by adjusting the antidiabetic therapy36 (although the fasting periods may be a challenge). Similarly, it has been suggested that patients with preexisting pulmonary disease should not receive dasatinib because of the risk of developing pleural effusions. I do not share this view, because there is no evidence that patients with pulmonary disease are more likely to develop pleural effusions; however, it would make sense to avoid dasatinib in patients with very poor lung function if there is concern about whether they will be able to tolerate a pleural effusion should it occur. The cardiac toxicity profile of the 3 drugs is similar, and there is no reason to prefer one drug over any other on cardiac grounds. Nilotinib should be avoided in patients with a history of pancreatitis. Nilotinib increases the serum triglyceride levels, so one may prefer to use another TKI in patients with dyslipidemia. Dasatinib has been shown to inhibit platelet function and has been associated with hemorrhaging,37,38 so I would favor the use of other TKIs in patients with platelet or clotting disorders. Patients of advanced age and patients with a history of autoimmune disease have a higher risk of developing pleural effusions on dasatinib,9,12 so it has been suggested that dasatinib should be avoided in this population.
A minority of patients present in advanced-phase disease. I treat these patients with dasatinib, although in this setting TKIs should be used as part of a more complex therapeutic strategy that includes conventional chemotherapy and eventually allogeneic stem cell transplantation if possible.
Some patients are diagnosed with CML while pregnant. There is limited experience using imatinib during pregnancy, and there is almost no relevant information on the use of nilotinib or dasatinib.21 I manage the uncomplicated chronic-phase patient with leukapheresis until the delivery. A detailed review of the various options for managing a CML patient during pregnancy or a CML patient already on a TKI who wishes to conceive is beyond the scope of this manuscript.
Are the new drugs a good value for money?
The answer depends very much on the premium price over imatinib that health-care providers or patients have to pay. The price of imatinib, dasatinib, and nilotinib varies substantially in different countries. On average, the price of dasatinib or nilotinib is between 1.5 and 2 times the price of imatinib (but not always: in the United Kingdom, for example, nilotinib and imatinib are of similar price). This difference is likely to become wider because imatinib will soon be available worldwide as a generic product. Are these drugs a good value for money? Allow me to do some “back of an envelope calculations” to answer this question. Let us use data from the ENESTnd trial, which showed the greatest difference in response rate between the new drug and imatinib, namely a 15 percentage point higher CCyR rate at 1 year in the nilotinib arm. If we treat 100 patients with nilotinib, at 1 year, 80 will be in CCyR, of whom 65 would have achieved the same response if treated with imatinib. An additional 20 patients will fail to respond and presumably would also not have responded to imatinib. In other words, for each patient who benefits from the use of the more expensive drug, we need to treat 5.6 patients at a premium price who do not obtain additional benefit. This is indeed an oversimplification, but it does give some idea of the unnecessary added cost of the newer therapies, which, depending on the differences in prices, could be enormous. It is therefore easy to understand why health-care providers may seek therapeutic strategies in which most of the patients receive imatinib and the more expensive drugs are made available only to those who really need them.
Some final considerations and what I do in my clinic
None of the available TKIs is dramatically better than the others, so if you have a preferred drug, there is no reason to change your practice.
I am not worried about the pleural effusions caused by dasatinib. They are very easy to manage. The dasatinib can be reintroduced at the same or at a lower dose or patients can receive another TKI afterward.
There are emerging side effects for nilotinib (eg, progressive peripheral arterial occlusive disease and arteriosclerosis) and dasatinib (eg, pulmonary hypertension). The frequency of these side effects seems to be very low and, in my opinion, there is no reason for alarm. Having said that, it is important to keep an open mind, because the full side-effect profile of these drugs is not yet fully established.
Dasatinib and nilotinib induce CCyR in a higher proportion of patients than imatinib, but 2 years down the line, the proportion of patients who have failed therapy is very similar, so it probably does not make much difference which TKI you choose as initial therapy.
Possibly the main advantage of using dasatinib or nilotinib as initial therapy is that they may reduce the number of patients who progress early after diagnosis, although this has not yet been proven fully. Strategies of early intervention may reduce the early progression in imatinib-treated patients, so this difference may not stand the test of time.
The treatment can be changed if a patient suffers side effects that affect his or her quality of life. Our experience with changing drugs in responding patients due to side effects is very positive.
In conclusion, my personal impression is that does not matter which TKI one chooses for initial treatment of CML in the chronic phase. What does matter is to have the right therapeutic strategy, which is based on 2 principles, early assessment of response and swift management of side effects, changing the TKI when required. So how does one choose the initial therapy? In those settings in which nilotinib and dasatinib are more expensive than imatinib, the choice is easy: imatinib. The current data do not justify paying a premium price for any of the TKIs. If money is not a factor, I prefer to use one of the second-generation TKIs in part because they have the glamour of novelty. I also believe that both nilotinib and dasatinib have less “insidious toxicity” and that they are more efficacious than imatinib (although the latter is yet to be proven). Major centers have reported extraordinarily good results with both drugs in series smaller than the large randomized trials.39,40 My first choice is therefore either dasatinib or nilotinib. Sometimes there is a clear reason (see above) to choose one drug over the other, but it is very important to discuss the pros and cons of both of these drugs with the patient and then together choose the drug that seems more suitable for the lifestyle and concerns of each individually. Some patients may prefer imatinib because we have much more experience with this drug. Whatever the choice, it is of paramount importance to assess the response early and to act accordingly. It is also important to monitor for side effects and take appropriate measures, including changing medication when necessary.
Acknowledgments
The author thanks Prof John Goldman, Dr Ed Kanfer, Dr Dragana Milojkovic, and Prof Katy Rezvani for critical review of this manuscript.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Novartis and BMS. Off-label drug use: None disclosed.
Correspondence
Dr David Marin, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; Phone: 44-20-8383-1627; Fax: 44-20-8383-8575; e-mail: d.marin@imperial.ac.uk.