Abstract
A 24-year-old man from Ecuador presents to your clinic with dyspnea on exertion, bruising, and petechiae. He is noted to be pancytopenic with ANC 430, hemoglobin 7.4 g/dL (reticulocyte count 0.9%), and platelets 18 000. His BM biopsy is hypocellular for age. Ultimately, he is diagnosed with severe aplastic anemia. He is the only child of 2 South American parents without any matches in the unrelated donor registry, including cord blood. He is red cell– and platelet transfusion–dependent. He has been recommended therapy with antithymocyte globulin and cyclosporine but declined it. He seeks recommendations about new alternatives to this regimen to improve his chance of response.
Severe aplastic anemia (SAA) is a rare and life-threatening disorder characterized by peripheral blood cytopenias and a hypocellular BM.1 Allogeneic hematopoietic cell transplantation is the mainstay of therapy for patients < 40 years of age. Because < 30% of patients have an appropriate HLA-compatible donor, research efforts are focused on immunosuppressive treatments as first-line therapy to improve outcomes. Antithymocyte globulin (ATG) and cyclosporine (CsA) have been the standard initial regimen for nearly 2 decades, with response rates from 50%-70%, and this remains the current recommendation for initial therapy for SAA.2
To examine the current best evidence of the various alternative regimens to ATG/CsA as a first-line therapy for severe aplastic anemia, we performed a comprehensive computerized literature search of the PubMed database combining the MESH terms “treatment of severe aplastic anemia” (6542 hits) AND “immunosuppressive therapy” (1767 hits) AND “not transplant” (199 hits) from 2005 through 1 June 2011. References chosen for discussion here include one systematic review on ATG/CsA,3 2 randomized controlled trials,4,5 3 observational studies,6–8 and one meta-analysis.9 Review of the references listed in a recent review article10 added one additional pilot study.11
The study of the addition of sirolimus to ATG/CsA was found to have a lower overall response rate at 6 months than ATG/CsA alone (51% vs 62%).4 Another study of the addition of mycophenolate mofetil to ATG/CsA with a 62% overall response rate also did not show improvement over standard ATG/CsA.12 A systematic review of the addition of growth factors to standard therapy showed no difference in overall response at 12 months or in overall mortality.9 Alternative therapies to ATG/CsA have also been explored and demonstrated no benefit. Tacrolimus in place of the CsA with ATG was tested in a small study of children and found to be equivalent, with a complete response of 88% for tacrolimus and 85% for CsA. Alemtuzumab was also used as first-line therapy and found to have a total response rate of 58%.11 Androgens were a mainstay of SAA therapy before ATG/CsA and are still used if ATG/CsA not available. However, the overall response rate in children was reported to be only 46%.7 Finally, high-dose cyclophosphamide has been evaluated with ATG and alone. The randomized controlled trial5 showed no difference in response, but demonstrated higher mortality with cyclophosphamide. The observational study showed an overall response rate of 77%,8 but there was no control for comparison (Table 1).
Regimens reviewed
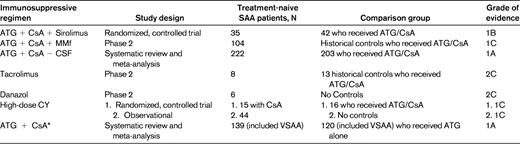
ATG indicates antithymocyte globulin; MMF, mycophenolate mofetil; CY, cyclophosphamide; and VSAA, very severe aplastic anemia.
*This meta-analysis also included patient with non-severe AA and these patients are not included here.
The addition of drugs to the standard ATG/CsA regimen or alternative regimens is routinely reported in the literature as novel therapy for SAA. In clinical practice, there is the admirable desire to improve a patient's response rate, and these agents are often administered despite little evidence to suggest that they are improvements over the standard of care and some evidence of detriment.
Based on this review, we conclude that multiple regimens for SAA have been explored as alternatives to ATG/ CsA, without evidence of benefit in response rates or overall survival. This conclusion is based on both randomized controlled trials and small observational series. Therefore, based on the highest grade of evidence3 (grade 1A), ATG/CsA alone is the only appropriate first-line therapy that can be recommended to the patient described above.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: Very few of the drugs used to treat aplastic anemia are approved for that purpose (eg, cyclosporin and cyclophosphamide). Off-label drug treatments for aplastic anemia are discussed in a research context but are not endorsed.
Correspondence
Amy E. DeZern, Department of Oncology, The Johns Hopkins University School of Medicine, CRB 1, Room 186, Baltimore, MD 21205; Phone: (410) 614-4459; Fax: (410) 955-0185; e-mail: adezern1@jhmi.edu.
