Abstract
Interindividual variability in the pharmacological response to antiplatelet drugs has been reported in some studies. Suboptimal response to aspirin, as determined by specific tests (serum thromboxane B2), appears to be rare and in most cases is caused by poor compliance. In contrast, studies using specific tests to measure the pharmacological effect of clopidogrel showed a wide variability of responses, with a significant number of subjects (approximately one-third) who were very poor responders. Interindividual differences in the extent of metabolism of clopidogrel to its active metabolite is the most plausible mechanism for the observed interindividual variability in platelet inhibition. Tailored treatment based on laboratory monitoring of platelet function has been proposed as a solution to poor responsiveness to clopidogrel. However, we still need to identify the ideal laboratory test and to answer basic questions on its clinical utility and cost-effectiveness before monitoring clopidogrel therapy can be recommended in clinical practice.
Introduction
Antiplatelet drugs are widely used to decrease the risk of major adverse cardiovascular events (MACEs). The main antiplatelet agents that are currently used in clinical practice are acetylsalicylic acid (aspirin), which irreversibly inhibits the cyclooxygenase-1 (COX-1)–dependent production of thromboxane A2 (TxA2); the thienopyridines, which irreversibly inhibit the platelet P2Y12 receptor for ADP; and inhibitors of the glycoprotein complex IIb/IIIa (integrin αIIbβ3).
Since their introduction into clinical practice, antiplatelet drugs have been administered to patients at standard doses without monitoring their pharmacological effects by laboratory tests. However, in the last few years, several studies revealed interpatient response variability to aspirin and the thienopyridine drug clopidogrel. Because poor responders are not adequately protected from MACEs, it has been proposed that antiplatelet therapy should be tailored to each individual patient based on the results of platelet function tests. Although this approach is generally considered a desirable evolution of modern medicine, which ideally should be tailored based on individual needs, it is in fact an old remedy (of yet unproven efficacy in the case of antiplatelet therapy) to the problem of response variability to antithrombotic drugs. Treatment with vitamin K antagonists and with unfractionated heparin has always been tailored to the individual patient based on laboratory monitoring, because the bioavailability of these drugs is unpredictable and highly variable. However, laboratory monitoring is expensive, increases the workload of health personnel, may be inaccurate, is uncomfortable for patients, and may decrease patients' adherence to treatment.1 For the above reasons, treatment plans involving anticoagulant drugs are progressively disposing of laboratory monitoring, thanks to the introduction into clinical practice of new drugs with very good and predictable bioavailability such as low-molecular-weight heparins, which do not need laboratory monitoring and have progressively replaced unfractionated heparin. Therefore, it appears that antiplatelet therapy is heading in the opposite direction of anticoagulant therapy. This review focuses on the magnitude, causes, clinical consequences, and management, if any, of response variability to antiplatelet therapy
How to measure the pharmacological response to antiplatelet drugs
As already mentioned, both aspirin and the thienopyridines selectively inhibit a single pathway of platelet activation; their good antithrombotic effect is explained by the fact that both the TxA2 pathway and the ADP pathway contribute to the amplification of platelet activation and are essential for the full aggregation response of platelets.2
Many studies have used various techniques to measure platelet function ex vivo to evaluate the degree of its inhibition by antiplatelet treatment and, in some instances, to predict the risk of atherothrombotic events.2 Although this approach is justified and rational, one should be aware that the relative importance of the TxA2 and P2Y12 pathways in platelet activation varies considerably among different subjects and with the type of laboratory test used.2 Therefore, the finding of high residual platelet reactivity in vitro in patients on aspirin or clopidogrel may not necessarily imply that these patients are resistant to treatment, especially when platelet function is measured by laboratory tests that are not specific for the effect of the antiplatelet drug on its pharmacological target. Therefore, only the use of specific tests that measure the pharmacological effect of the antiplatelet drug will clarify whether platelet hyperreactivity is due to insufficient pharmacological effect of the drug or to other causes.2
Laboratory tests to measure the response to aspirin
Light-transmission aggregometry is a poorly standardized technique that is sensitive to several variables.3,4 Even when arachidonic acid, the precursor of TxA2, is used as a platelet agonist, the results obtained with this technique may overestimate the prevalence of poor responders to aspirin.3 Methods that measure directly the capacity of platelets to synthesize TxA2 are certainly preferable; of these, urinary levels of the TxB2 metabolite 11-dehydrothromboxane B2 represent a time-integrated index of TxA2 biosynthesis in vivo.3 However, approximately 30% of the urinary metabolite derives from extra-platelet sources,5 and this fraction can increase in pathological conditions such as inflammatory diseases.3 Therefore, the method is not highly specific for monitoring the effects of aspirin on platelet COX-1. In contrast, serum TxB2 reflects the total capacity of platelets to synthesize TxA2, of which it is a stable metabolite. Because the contribution of other blood cells to its synthesis is marginal, serum TxB2 is the most specific test to measure the pharmacological effect of aspirin on platelets.2
Comparison of different laboratory methods have usually revealed very weak or no correlation,6 indicating that they are sensitive to different parameters. Usually, the number of individuals with residual, significant TxB2 production on aspirin was extremely low, whereas the prevalence of individuals with no inhibition of platelet function measured by other, less specific tests tended to be much higher.2
Laboratory tests to measure the response to inhibitors of P2Y12
ADP-induced platelet aggregation measured by light-transmission aggregometry may overestimate the prevalence of poor responders to P2Y12 inhibitors, because ADP induces platelet aggregation by interacting with both of its platelet receptors, P2Y1 and P2Y12. The platelet aggregation–based assay VerifyNow P2Y12 and the flow cytometry–based assay Platelet VASP (which measures the P2Y12-dependent inhibition by ADP of phosphorylation of vasodilator-stimulated phosphoprotein) are more specific assays for measuring the effects of thienopyridines and other drugs inhibiting the platelet P2Y12 receptor.3
Interindividual variability of response to aspirin
Studies measuring serum TxB2 to assess the response to aspirin have revealed that the prevalence of poor responders is extremely low,2,5,6 with the possible exceptions of patients who have recently undergone coronary artery bypass graft surgery7 and those with myeloproliferative neoplasms,8 in whom high levels of serum TxB2 despite aspirin treatment can occasionally be measured. Although TxB2 production by platelets from aspirin-treated diabetic patients may be slightly higher than that by normal, aspirin-treated platelets, the degree of its inhibition by aspirin is usually still extremely good.9,10
Causes of altered pharmacological response to aspirin
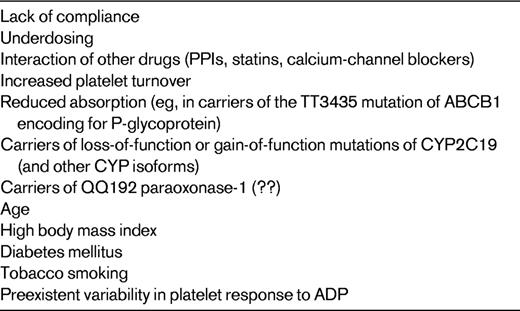
Lack of compliance is probably the most frequent and plausible cause of insufficient inhibition of COX-1 by aspirin (Table 1).3,5,11,12 As of yet, data for the extent of the genetic contribution to the pharmacological response to aspirin are inconclusive, with investigators reporting conflicting results.13 Increased platelet turnover in some disease states could account for a more rapid recovery of COX-1–dependent platelet function within the 24-hour interval between 2 consecutive administrations of the drug (Table 1).14 Competition of aspirin with other nonsteroidal anti-inflammatory drugs such as ibuprofen can prevent aspirin access at Ser529 of COX-1 and, as a consequence, its irreversible acetylation and inactivation of the enzyme (Table 1).15
Clinical consequences of altered pharmacological response to aspirin
Impaired inhibition of urinary excretion of Tx metabolites has been associated with increased incidence of MACEs, and is thought to be due to insufficient inhibition of platelet COX-1.16 However, considering that atherothrombosis is an inflammatory disease, a possible alternative interpretation of these results is that high urinary levels of 11-dehydrothromboxane B2 reflect an increased generation of COX-2–dependent prostaglandins and thromboxanes by monocytes/macrophages in severely inflamed atherosclerotic plaques, which are at high risk of thrombotic complications.
The degree of inhibition of MACEs by aspirin in diabetic patients does not seem to be significantly lower than in nondiabetic patients, both in primary17 and secondary prevention,18 indicating that the slightly lower degree of inhibition of TxB2 production by aspirin that has been occasionally described in diabetic platelets compared with nondiabetic platelets is not biologically or clinically relevant.
Frelinger et al reported that a direct measure (serum TXB2 < 3.1 ng/mL) but not indirect measures of residual platelet COX-1 function are associated with subsequent MACEs in aspirin-treated patients. However, given a potential for bias based on the method used to define the cutoff for high residual serum TXB2, the need to adjust for covariables to show a significant association between serum TXB2 and subsequent MACEs, and the failure of indirect assays of residual platelet COX-1 function to confirm an association with MACEs, the link between residual platelet COX-1 function as reported by serum TXB2 < 3.1 ng/mL and MACEs should be further verified.19
Management of response variability to aspirin
Because insufficient response to aspirin is extremely rare and its proper treatment, if any, is unknown, it is usually recommended that the response to aspirin in treated patients should not be tested other than in research studies.2,3,6,20 The most important factor contributing to these recommendations is that no published studies have addressed the clinical effectiveness of altering therapy on the basis of the results of laboratory tests. Moreover, it is known that increasing aspirin dosage would not be very effective in decreasing the incidence of MACEs, but is associated with increased risk of bleeding.21
Interindividual variability of response to clopidogrel
In contrast to aspirin, studies using specific tests to measure the pharmacological effect of clopidogrel did show a wide variability of responses, with a significant proportion of subjects (approximately one-third) who were very poor responders, displaying almost no inhibition of platelet function.22,23
Causes of poor response to clopidogrel
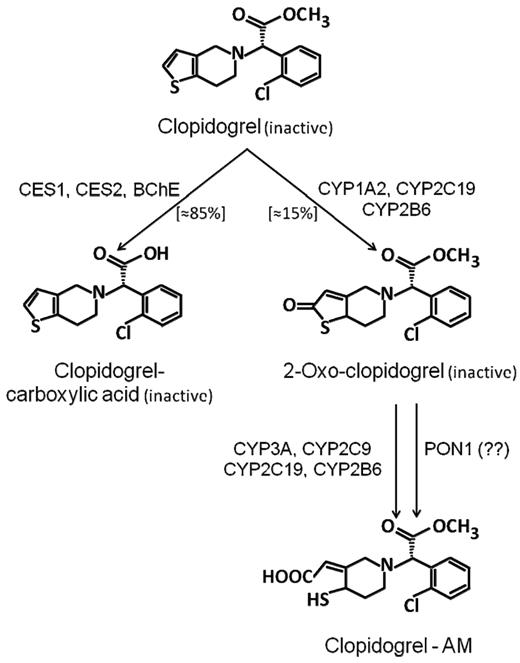
Clopidogrel is an inactive prodrug, which, to exert its antiplatelet effect, needs to be metabolized to its active metabolite by hepatic cytochrome P450 (CYP) isoenzymes in a 2-step process: the isoforms CYP2C19, CYP1A2, and CYP2B6 are responsible for the first metabolic step, whereas CYP2C19, CYP2C9, CYP2B6, and CYP3A are responsible for the second step (Figure 1).22,23 Several lines of evidence strongly suggest that variability in active metabolite generation is the primary explanation for clopidogrel antiplatelet response variability (Table 2).22,23 Loss-of-function mutations (eg, CYP2C19*2) and gain-of-function mutations (eg, CYP2C19*17) of CYP isoforms are associated with variable degrees of production of the active metabolite, and therefore of the pharmacodynamic response to the drug.22,23 A recent study identified paraoxonase-1 (PON1) as the crucial enzyme for clopidogrel bioactivation (Figure 1), with its common Q192R polymorphism determining the rate of active metabolite formation, and failed to show any influence of CYP2C19*C on the pharmacological response to clopidogrel.24 However, a subsequent study in 1524 patients undergoing percutaneous coronary intervention did not confirm these findings, demonstrating that CYP2C19*C, but not PON1 Q192R, is negatively associated with the platelet response to clopidogrel.25
Metabolism of clopidogrel. Schematic representation of the main enzymatic pathways involved in the metabolism of clopidogrel. The role of PON-1 in clopidogrel metabolism is still controversial. CYP indicates cytochrome P450 isoenzyme; PON, paraoxonase; ES, carboxylesterase; BChE, butyrylcholinesterase.
Metabolism of clopidogrel. Schematic representation of the main enzymatic pathways involved in the metabolism of clopidogrel. The role of PON-1 in clopidogrel metabolism is still controversial. CYP indicates cytochrome P450 isoenzyme; PON, paraoxonase; ES, carboxylesterase; BChE, butyrylcholinesterase.
Variable levels of active metabolite generation and/or of pharmacodynamic response to clopidogrel are also associated with: (1) limited intestinal absorption, which is associated with the homozygous 3435C → T mutation of ABCB1, a gene encoding for the efflux pump P-glycoprotein, a key protein involved in thienopyridine absorption; (2) interaction with other drugs, including proton pump inhibitors (PPIs), calcium-channel blockers, and statins, which are metabolized by CYP2C19 and CYP3A isoenzymes; (3) stimulation of CYP1A2 activity by tobacco smoking; (4) preexistent variability in platelet response to ADP (Table 2).22,23 Other variables that influence the response to clopidogrel include advanced age, high body mass index, and diabetes mellitus, which are associated with decreased response to the drug (Table 2).22,23 Finally, noncompliance has to be considered an obvious and frequent cause of poor response to clopidogrel.3
Clinical consequences of poor pharmacological response to clopidogrel
Several independent studies have demonstrated an association between suboptimal generation of the active metabolite, decreased inhibition of platelet function, presence of enzyme polymorphisms, and clinical outcomes.22,23 However, no study has yet associated all of these parameters in the same patient population, and some uncertainties still persist.
The association between poor clinical outcomes of patients on treatment with clopidogrel and the presence of loss-of-function mutations of CYP has been demonstrated by several observational and intervention studies. Two recent meta-analyses demonstrated an increased risk of MACEs, particularly of stent thrombosis, in carriers of either 1 or 2 mutated CYP2C19*2 alleles.26,27 The question remains open as to whether this association is explained by impaired clopidogrel metabolism by carriers of the mutation or by pleiotropic effects of the mutation with a negative impact on cardiovascular outcomes independently of the administration of clopidogrel. This latter hypothesis is corroborated by the results of a retrospective analysis of 2 trials comparing clopidogrel with placebo in patients with acute coronary syndromes and in patients with atrial fibrillation, which showed that the protective effect of clopidogrel compared with placebo is consistent, irrespective of CYP2C19 loss-of-function carrier status.28 However, the finding that CYP2C19 loss-of-function carrier status is not negatively associated with clinical outcomes in patients treated with the new thienopyridine prasugrel (the metabolism of which is less dependent on CYP2C19 function than clopidogrel)29 or ticagrelor (a direct and reversible P2Y12 inhibitor)30 or in patients not on treatment with anti-P2Y12 drugs31 does not support the hypothesis of pleiotropic effects of mutant CYP2C19.
In patients treated with clopidogrel, ABCB1 3435C → T genotype was significantly associated with the risk of cardiovascular death, myocardial infarction, or stroke in the TRITON TIMI-38 trial comparing clopidogrel and prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention (PCI).31 TT homozygotes had a 72% increased risk of the primary end point compared with CT/CC individuals. In contrast, ABCB1 genotypes were not significantly associated with clinical outcomes in patients treated with prasugrel.32
PON-1 QQ192 homozygous individuals were found to be at considerably higher risk than RR192 homozygous individuals of stent thrombosis, lower PON1 plasma activity, lower plasma concentrations of the active metabolite of clopidogrel, and lower platelet inhibition.24 However, these findings were not confirmed by a subsequent study of 1594 patients undergoing PCI, which failed to show an interaction between PON1 QQ192 and stent thrombosis.25
No clear negative association between the coadministration of clopidogrel with lipophilic statins and calcium-channel blockers with clinical outcomes has been documented so far, despite their negative interaction with the pharmacodynamic response to the drug.23
Two meta-analyses of observational studies, case-control studies, and post hoc analyses of randomized clinical trials showed that the risk of MACEs was higher in patients on combined treatment with clopidogrel and PPIs compared with patients on clopidogrel not in combined treatment with PPIs.27,33 However, a large observational study showed that the high risk of MACEs was associated with PPI administration independently of whether PPIs were coadministered with clopidogrel, suggesting that PPIs may have a negative impact on clinical outcomes independently of their potential interaction with the metabolism of clopidogrel.34 Moreover, the only randomized, placebo-controlled clinical trial that was designed to test prospectively the interaction between clopidogrel and omeprazole, which was terminated before completion of the randomization, failed to show that the coadministration of omeprazole and clopidogrel increases the incidence of MACEs.35
In conclusion, although it is plausible and likely that suboptimal pharmacodynamic response to clopidogrel is associated with poor clinical outcomes, there still are contrasting reports in the literature linking negative interactions with the pharmacodynamic response to the drug and negative interactions with clinical outcomes.
Management of response variability to clopidogrel
Tailored treatment of patients based on the results of platelet function tests or of CYP genotyping has been proposed to solve the problem of clopidogrel resistance.22,23 However, this approach cannot be recommended in daily clinical practice yet, because the best laboratory method to monitor the effects of clopidogrel on platelet function still needs to be identified, standardized (for pre-analytical and analytical variables), and validated in the clinical setting.22 Several recent studies have demonstrated that the agreement among different laboratory tests to identify poor responders is rather low and that the assessment of platelet response to clopidogrel is highly test specific.22 Moreover, loss-of-function mutations of CYP account for only ∼10% of the variability of response to clopidogrel,36 thus explaining the high degree of inaccuracy of CYP genotyping in predicting the response to clopidogrel.22 In addition, preliminary experiments evaluating the effects of increasing the dose of clopidogrel in resistant patients yielded results that were unsatisfactory, because many patients remained “resistant” to clopidogrel even after repeated administrations of high doses of the drug.37
Mostly based on the aforementioned considerations, and in compliance with the rules of evidence-based medicine, a recent consensus paper concluded that until the results of large-scale trials of personalized antiplatelet therapy are available, the routine use of platelet function measurements in the care of patients with cardiovascular disease cannot be recommended.23
Our incompetence on personalized antiplatelet treatment has been recently confirmed by the negative results of GRAVITAS, the first, large randomized prospective trial testing the efficacy and safety of personalized clopidogrel treatment in patients undergoing PCI.38 GRAVITAS showed that, in patients with high platelet reactivity on clopidogrel (≥ 230 PRU with the VerifyNow-P2Y12 test, 12-24 hours after PCI), high-dose clopidogrel (an additional 600 mg followed by 150 mg daily) did not reduce the incidence of MACEs nor did it increase the incidence of bleeding compared with the standard dose (75 mg daily).38 Therefore, the adoption of the personalized treatment strategy that has been tested in GRAVITAS would cause—and has already caused in the institutions that have adopted it—an unjustified expenditure of resources without being of any benefit to the patients.
Different strategies of personalized treatment (serial testing, combined patient genotyping and serial testing, use of the new P2Y12 inhibitors instead of high-dose clopidogrel in low responders) might prove effective.38,39 However, serial testing with or without genotyping will increase the overall cost of treatment, possibly offsetting the advantage of using the cheaper drug clopidogrel instead of the newer, more expensive P2Y12 inhibitors. In addition, tailored antiplatelet treatment should not be considered an achievement of modern medicine to be pursued by any means, but rather a potential solution to the problem of hyporesponsiveness to clopidogrel: it is quite obvious that not having to face the problem would be preferable. Finally, the use of the new P2Y12 antagonists prasugrel or ticagrelor instead of clopidogrel would eliminate the problem of hyporesponsiveness because they effectively inhibit platelet function in the majority of patients.22 Both prasugrel and ticagrelor increase the incidence of bleeding, but this is mostly because all patients treated with these drugs display a good inhibition of platelet function, and all are therefore protected from thrombosis and exposed to the risk of bleeding (as opposed to only ∼ 70% of patients treated with clopidogrel).22 Accordingly, it has been clearly demonstrated that patients displaying good response to clopidogrel are at higher risk of bleeding than those who are nonresponsive.22 If all patients were to respond well to clopidogrel (which is the aim of tailored treatment with clopidogrel), they would have a lower incidence of MACEs and a higher incidence of bleeding, exactly like prasugrel- or ticagrelor-treated patients. The rate of bleeding complications is related to the degree of inhibition of platelet function rather than to the type of drug used to cause it. The use of the new P2Y12 inhibitors in all patients without testing might prove to be more effective and cost-effective than personalized treatment, but this hypothesis should be tested in controlled studies. While we await the results of additional controlled studies, personalized treatment should not yet be implemented in clinical practice.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from AstraZeneca and has received honoraria from Eli Lilly, Daiichi Sankyo, and AstraZeneca. Off-label drug use: None disclosed.
Correspondence
Marco Cattaneo, MD, Unità di Medicina 3, Ospedale San Paolo Università degli Studi di Milano, Via di Rudinì 8, 20142 Milano, Italy; Phone: 39-0250323095; Fax: 39-0250323089; e-mail: marco.cattaneo@unimi.it.