Abstract
Whereas RBC transfusion therapy is lifesaving in thalassemia, obligatory iron loading accompanies such treatment and chelation therapy to remove and detoxify iron resulting from these chronic transfusions must therefore be administered. Morbidity and mortality in thalassemia is linked closely to the adequacy of chelation. Three chelators are currently available worldwide—deferoxamine, deferasirox, and deferiprone, although the latter is available in North America only in research protocols and compassionate use programs. These chelators can be used as monotherapy or in combination, although only the combination of deferiprone and deferoxamine has been extensively studied to date. Several factors, including chelator availability and its properties, drug tolerability, degree of organ-specific iron loading, ongoing transfusional iron burden, and patient preference, must be considered in the design of optimal, individualized chelation regimens, and these factors must periodically be reviewed and chelation adjusted accordingly. Ultimately, comparative effectiveness trials may help to determine the ideal strategy (eg, intensification of monotherapy or combined therapy including agents and doses) for treating various scenarios of organ-specific iron loading.
Introduction
The majority of data regarding the treatment of transfusional iron overload are derived from the thalassemia population. Although this information is often extrapolated to other chronically transfused populations, clear differences exist. For example, iron-related cardiac morbidity and endocrinopathies, as well as cardiac and pancreatic iron loading, appear to be lower in sickle cell disease compared with thalassemia.1,2 In addition, susceptibility to chelator toxicities may be influenced by the underlying blood disorder. In particular, patients with bone marrow failure syndromes such as Diamond Blackfan anemia may be at higher risk of developing neutropenia with deferiprone.3 This review is intended to provide an overview of the approaches to chelation therapy in patients with transfusion-dependent thalassemia.
Goals of chelation
Multiple methods of assessing the degree of iron overload exist, including serum ferritin levels, liver iron concentration by biopsy, superconducting quantum interference device (SQUID), and magnetic resonance imaging (MRI), and cardiac iron loading assessed by MRI. Each method has benefits and limitations, and often combinations of these tests are used to monitor iron burden. Details about MRI techniques for estimation of iron burden are provided in an accompanying article in this section and will not be discussed herein.
The overall goal of chelation is to maintain a “safe” iron level among patients who have increased iron loading, typically from transfusion therapy. Ideally, chelation should be administered in a way to prevent excess iron accumulation and the resulting hepatic, endocrinologic, and cardiac complications by matching the ongoing transfusional iron intake. Chelators also bind non-transferrin-bound and labile plasma iron, protecting vulnerable organs such as the heart from damage by these toxic iron forms, and continuous chelator exposure is optimal in this regard. In practice, however, chelation therapy is often used to remove excess stored iron and to reverse related complications.
The level of “acceptable” iron burden to maintain in chronically transfused individuals through the use of chelation has recently been called into question.4 In untransfused individuals, the normal liver iron concentration ranges from 0.17-1.8 mg Fe/g dry wt. Traditionally, chelation therapy has been dosed to maintain a slightly higher liver iron concentration of 3 ≤ 7 mg Fe/g dry wt of liver, a level of hepatic iron burden that is seen in asymptomatic heterozygotes for hereditary hemochromatosis.5 Corresponding ferritin goals are to maintain a level between 500 and 1500 ng/mL. In recent years, however, more aggressive chelation regimens have been advocated, typically with combination deferiprone (75-100 mg/kg/d) and deferoxamine (20-60 mg/kg/d) aimed at achieving “normal” body iron stores. These regimens are accompanied by a subsequent reduction in the frequency and dose of deferoxamine while maintaining these “normal” iron levels to reduce the risk of chelator toxicity.6 With such an approach, 15 of 18 patients with cardiac dysfunction developed normal cardiac function, including 9 of 12 who had previously required cardiac medications and were able to discontinue them. In addition, no deterioration in cardiac function was seen in the 34 patients with normal cardiac function at baseline.6 Furthermore, endocrinopathies including hypothyroidism, hypogonadism, and non-insulin-dependent glucose intolerance were reversed in some, but not all, patients treated in this fashion.6
Nonetheless, the risk of toxicity with deferoxamine is increased among patients with lower iron burdens,7 and bone changes and growth failure can develop in children when iron burden is low compared with the chelator dose,8 calling into question whether such an aggressive approach to chelation is appropriate. It is less clear if similar toxicity is seen with deferasirox or deferiprone at low levels of iron burden. Audiological and ophthalmological toxicity were uncommon among subjects maintained on deferiprone monotherapy after achievement of normal body iron stores, suggesting that this chelator may be safer to use at lower iron levels.6 Similarly, a preliminary report showed no increase in adverse effects among subjects taking deferasirox whose serum ferritin level fell below 1000 ng/mL9 ; however, current prescribing guidelines recommend holding the drug when ferritin levels are below 500 ng/mL.
Chelator properties
The properties of the 3 currently available chelators are summarized in Table 1. A brief description of each chelator's properties is presented below, with emphasis on data regarding the ability to remove cardiac iron.
Properties of iron chelators
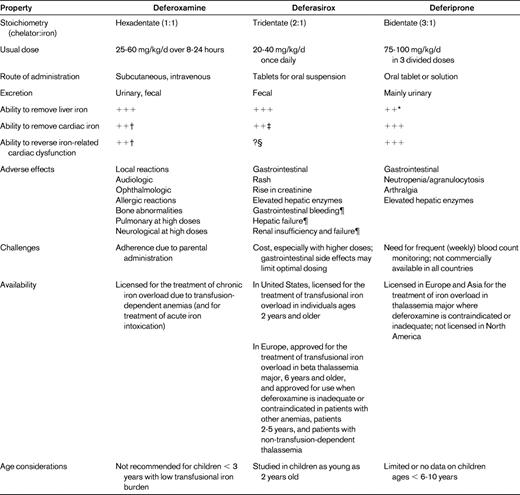
*Reports of insufficient liver iron removal in some patients at doses of 75 mg/kg/d, but higher dosing, especially for subjects with high transfusional iron burden, may be more effective
†With continuous infusion.
‡Limited data show cardiac iron removal with modest cardiac iron loading; may be less effective in patients with more severe hepatic iron loading. Further studies are needed to determine effectiveness at cardiac iron removal compared with deferoxamine and deferiprone.
§Not studied in patients with cardiac dysfunction. Lack of improvement in ejection fraction with deferasirox treatment (for 1-2 years) in patients with normal cardiac function.
¶More common in patients with advanced age, high-risk myelodysplastic syndrome, underlying hepatic or renal insufficiency, and/or cytopenias.
Deferoxamine
Deferoxamine, the first available chelator, has now been used in routine clinical practice for more than 40 years. Clear data support its effectiveness in lowering serum ferritin levels and hepatic iron10,11 and in preventing endocrine complications.12,13 Long-term therapy with deferoxamine is also associated with a reduction in cardiac complications and improved survival.14 In addition, deferoxamine, usually given in higher doses of up to 60 mg/kg/d as a continuous intravenous infusion, can reverse cardiac complications15 and reduce cardiac iron burden as measured by T2* MRI.16 The adverse effects of deferoxamine are listed in Table 1. Redness and induration at the infusion site are the most common. Audiological, ophthalmological, growth, and bone toxicities may be minimized by avoiding “overchelation.” However, the greatest challenge with deferoxamine is patient adherence with therapy, because the need for parental administration is cumbersome, uncomfortable, inconvenient, and time-consuming. Furthermore, cardiac morbidity and mortality continue to occur in patients treated with deferoxamine, likely related to difficulties with adherence.17
Deferiprone
Deferiprone, introduced into clinical trials in the 1980s, was the first oral chelator to undergo extensive testing. Studies have shown a reduction or stabilization of serum ferritin levels and liver iron concentrations in most, but not all, patients with transfusional iron overload.18 Some of the failures may be attributed to underdosing of the drug, and higher doses of 90-100 mg/kg may be more effective in patients with high transfusional iron intake and/or elevated body iron stores.19 Deferiprone is a small molecule that is lipophilic, enabling entry into myocytes, and this chelator appears to be particularly efficacious with regard to cardiac iron removal. Retrospective studies have demonstrated reduced cardiac morbidity and mortality17,20 and lower myocardial iron deposition21 among patients treated with deferiprone compared with deferoxamine. Furthermore, in a randomized trial among patients with moderate cardiac siderosis (T2* = 8 < 20 ms) and normal cardiac function, a significantly greater improvement in cardiac T2* and left ventricular function was seen after treatment with deferiprone compared with deferoxamine.22 In a large clinical observational study, treatment with deferiprone resulted in improvement in cardiac T2* among patients with all degrees of cardiac iron loading (including severe iron loading with T2* < 8 ms).23 Treatment with deferiprone resulted in a significantly greater overall reduction in cardiac iron compared with deferasirox, although small numbers of subjects receiving deferasirox at relatively low doses (mean, 26.6 mg/kg/d) may have limited these analyses.23 The major challenge to treatment with deferiprone is the lack of commercial availability in North America; patients currently can only receive the drug through research protocols or compassionate use programs. Common adverse effects of deferiprone are presented in Table 1. The most serious adverse effects associated with deferiprone are agranulocytosis and neutropenia, with an incidence of 0.2 and 2.8 per 100 patient-years, respectively.24 Weekly blood counts are recommended, which can be inconvenient for patients.
Deferasirox
Deferasirox in doses of 20-30 mg/kg/d was shown to have similar efficacy to deferoxamine with regard to reduction in liver iron concentration and serum ferritin levels in a large randomized trial in thalassemia major.10 The ability to remove hepatic iron was demonstrated in several additional studies.25,26 In a single-arm trial of 101 patients with mild to severe cardiac iron loading treated with deferasirox at a mean dose of 34.5 mg/kg/d (range, 30-50 mg/kg/d), cardiac T2* significantly improved from a mean of 11.2 to 14.8 ms over 2 years of treatment; significant improvements were seen in both the group with severe (T2* > 5 < 10 ms) cardiac siderosis (7.3 to 8.7 ms) and those with mild to moderate (T2* = 10 < 20 ms) siderosis (14.7 to 20.1 ms).27 No significant improvement in left ventricular ejection fraction was seen after 2 years of treatment with deferasirox, although the ejection fraction was normal at baseline.27 In a second report of 22 patients with cardiac T2* < 20 ms treated for 18 months with deferasirox (mean end of trial dose, 33.3 mg/kg), cardiac T2* improved by 43.3% ± 18.8% in 13 “responders” but worsened by 15.2% ± 17.8% in 14 patients; the failure to respond was predicted by higher baseline liver iron concentration and ferritin levels.28 Similar to the previous study, no change in left ventricular ejection fraction was seen over the 18 months of treatment. Administration of deferasirox (mean dose, 27.6 mg/kg/d) has also been shown to prevent cardiac iron accumulation; in 78 patients with thalassemia without evidence of cardiac iron loading (cardiac T2* ≥ 20 ms), cardiac T2* did not worsen over 1 year of treatment (32 to 32.5 ms).29 Moreover, in that study, no subjects with normal cardiac T2* at baseline developed an abnormal value (T2* < 20 msec) over the follow-up period. Interestingly, in contrast to the subjects with low cardiac T2* values, a significant improvement in ejection fraction (from 67.7 ± 4.7% to 69.6 ± 4.5%) was seen in this group of patients with normal cardiac T2* at baseline.
The ability of deferasirox to reverse cardiac disease has not yet been investigated, because the prior studies all required normal heart function for inclusion. Reversal of heart failure with deferasirox treatment in a patient with thalassemia and transfusional iron overload has been reported.30 Further studies are needed to delineate better the effect of deferasirox on cardiac iron- and iron-associated cardiac dysfunction.
Common adverse effects with deferasirox are listed in Table 1. Gastrointestinal disturbances often improve with continued administration of the drug, but have led to discontinuation of therapy in some patients. Rare reports of fulminant hepatic failure have resulted in the recommendation to obtain liver function tests every 2 weeks for 1 month after starting therapy and then monthly thereafter. Elevations in serum creatinine occur in approximately 1/3 of subjects, but rarely reach the abnormal range10 ; kidney function should also be monitored monthly.
Starting chelation therapy
In thalassemia major, chelation therapy is usually initiated in children 2 years of age or older who have received 10 units of RBCs and/or have a serum ferritin level above 1000 ng/mL on at least 2 measurements (not obtained when ill).31 This level of iron overload typically occurs after 1-2 years of transfusions. A dose of deferasirox of 20 mg/kg/d or deferoxamine of 25-30 mg/kg/d is often used initially to avoid the risk of toxicity with “overchelation” in growing children. In Europe, deferasirox is approved as a second-line agent in children younger than 6 years (Table 1), and therefore deferoxamine is usually prescribed initially. In addition, oral administration may be difficult in young children due to behavioral issues, which may make deferoxamine a better choice for some children.32
Tailoring chelation regimens
Chelation regimens, including agent(s) and dosages, should be individualized to target organ–specific iron burden, to maintain a net negative iron balance (or neutral balance for patients without elevated iron stores), to limit toxicity, and to optimize adherence. Patient preference must also be addressed because this will affect adherence and ultimately the success of the therapy, as well as quality of life. Chelation strategies tailored to liver and cardiac iron burden with ongoing transfusion requirements,33 serum ferritin and ongoing transfusion burden,34 or serum ferritin level and cardiac T2*35 have been proposed. A summary of one approach to treatment based on hepatic and cardiac iron loading is presented in Table 2.
Strategy for adjusting chelation therapy targeting organ-specific iron loading*

DFO indicates deferoxamine; DFX, deferasirox; DFP, deferiprone.
See Table 1 and text for dose ranges. Address adherence concerns and discuss strategies to improve adherence. If escalation is required and patient is receiving maximal dose or cannot tolerate due to toxicity, consider switching agent or combination therapy.
†Close monitoring for adverse events with dose adjustments as needed; use lower doses for growing children to avoid bone and other toxicity.
‡Due to lack of data, the use of deferasirox in patients with low cardiac T2* and cardiac dysfunction is not recommended routinely.
Organ-specific iron burden
Cardiac complications remain the most common cause of death in transfused thalassemia patients,14 and a central goal of iron chelation regimens is to prevent or remove cardiac iron loading. Patients who are maintaining low iron stores (normal cardiac T2*, liver iron concentration < 7 mg/g dry wt) can be maintained on their current chelation regimen using continued monitoring of organ-specific iron burden and transfusion requirements to adjust dose (ie, increase for rising levels and reduce or hold dose for low iron burdens). Cardiac T2* values of 10 < 20 ms indicate mild to moderate iron loading and values < 10 ms indicate severe myocardial siderosis. Patients with very low T2* values of < 6 ms have a 47% chance of developing congestive heart failure within the next year.36 Therefore, low cardiac T2* values (< 20 ms) should trigger intensification of chelation regimens regardless of liver iron burden. The development of cardiac dysfunction or arrhythmia should also prompt intensification of chelation. Combination therapy with deferoxamine and deferiprone, if available, should be considered for patients with low cardiac T2* or iron-related cardiac disease (see below).
Liver iron concentrations > 15 mg/g dry wt predict a higher risk of cardiac disease and death37 and progression of hepatic fibrosis, which may be exacerbated by concomitant hepatitis C infection. Therefore, intensification of chelation is also indicated for such elevated liver iron levels. Similarly, intensification is indicated for a liver iron concentration above 7 mg/g dry wt that is not decreasing.
Iron is removed from different organs at different rates, and hepatic iron levels usually improve more rapidly than cardiac iron levels with intensification of chelation, so both hepatic and cardiac iron must be measured to optimize chelation. Changes in ferritin levels often parallel changes in liver iron concentration, although a variety of factors, including ascorbate deficiency and infection/inflammation, either decrease or increase ferritin levels. Nonetheless, serial ferritin levels, which can be obtained more easily than liver or cardiac iron estimation, may be used to assess trends in iron burden and to help adjust chelator dosing. Ferritin levels above 2500 ng/mL are associated with an increased risk of morbidity and mortality, and levels persistently above this value should trigger intensification of chelation. Periodic monitoring of cardiac and hepatic iron is still recommended if possible.
Transfusional iron burden
It is clear that ongoing blood transfusion requirements influence the chelator dose needed: patients with higher transfusional iron intake generally require higher chelator doses. With transfusional iron intake of more than 0.5 mg/kg/d, deferasirox doses of 20 mg/kg/d were ineffective in more than half of subjects, but doses of 30 mg/kg/d led to a reduction in liver iron concentration in 82%.38 Similarly, deferoxamine doses of 25 ≤ 35 mg/kg/d, 35 ≤ 50 mg/kg/d, and ≥ 50 mg/kg/d were effective in 17%, 52%, and 89% of patients, respectively, at this level of transfusional iron intake.38 Similar dose principles likely hold true for deferiprone.
Careful monitoring of transfusional iron intake is needed. Older children and adults with higher rates of iron loading (> 0.5 mg/kg/d) will likely need higher doses of chelator (deferasirox, 30-40 mg/kg/d; deferoxamine, 40-50 mg/kg/d). Investigation of the reason for higher transfusional needs, such as splenomegaly or red cell antibody, should also be considered.
Adherence/patient preferences
Any therapy will only be effective if the patient actually receives it, and deviations from the prescribed course of chelation adversely affect outcome. Adherence with deferasirox therapy has been reported to be better than with deferoxamine,39 and patients reported greater satisfaction and convenience with deferasirox compared with deferoxamine.40 A preliminary report from the Thalassemia Clinical Research Network (TCRN) showed deferasirox to be the most common chelator used (57% of subjects in participating sites).39 Nonetheless, almost 20% of patients in the TCRN chose to continue deferoxamine and never tried deferasirox. Furthermore, certain chelators cannot be used in some patients due to the development of significant toxicities such as gastrointestinal bleeding and hepatic or renal failure with deferasirox or agranulocytosis with deferiprone. Other adverse effects, such as gastrointestinal upset with deferasirox or infusional site reactions with deferoxamine, may simply not be tolerable to some patients. Therefore, it is important to address side effects and adherence as well as changes in iron burden regularly to adjust chelation regimens.
Monotherapy
Intensification of chelation can be achieved by increasing the daily dose (and/or the number of days of administration for deferoxamine) of an individual chelator if the patient is currently receiving less than the upper recommended dose. Care should be taken, especially with young children, not to exceed the therapeutic index of a chelator (too high a chelator dose compared with iron burden), because this is associated with an increased risk of audiological, ophthalmologic, and bone toxicities.7,8 Changing the chelator agent (eg, deferoxamine to deferasirox or vice versa) should be considered in patients who are prescribed the highest recommended dose but still have unacceptable iron burden. Strategies to optimize adherence to treatment must be addressed as well. Such strategies include regularly reviewing trends in iron burden with the patient, having the patient keep the medication near an item frequently used (such as a toothbrush), and having the patient set cell phone or watch alarms to alert them to take their medication.
In the presence of iron-related cardiac disease or abnormal cardiac T2* values, increasing both the deferoxamine dose and duration of exposure (ie, continuous infusion 7 days per week) is recommended to limit exposure to toxic, nontransferrin-bound iron forms. Such an approach can improve cardiac T2* and reverse cardiac disease.15,16 Very high dosing of deferoxamine (such as 10 mg/kg/h) has been used in the past.41 However, acute pulmonary and neurotoxicity may develop with higher doses,42,43 so generally doses of > 60 mg/kg/d should be avoided. Deferiprone, where available, should be administered at doses of up to 100 mg/kg/d if used as monotherapy22 and preferably in combination therapy (see below), because data regarding the use of deferiprone as monotherapy with severe myocardial iron loading and cardiac dysfunction are limited. With cardiac iron loading (T2* < 20 ms), intensification of deferasirox therapy to 40 mg/kg/d also may be considered, but this approach cannot yet be routinely recommended for patients with iron-related cardiac dysfunction given the lack of data on the ability of deferasirox to improve cardiac function, especially in patients with low ejection fractions. Doses of deferasirox of > 30 mg/kg/d did not have apparent increased toxicity.44
Monotherapy is not effective in all patients for a variety of reasons. Adverse drug effects may prevent optimal dosing, poor adherence to treatment may lead to underdosing, and/or individual pharmacokinetics45 may result in an inadequate response. Combination therapy may be effective in such situations and particularly when dangerously high levels of cardiac iron exist.
Therapy with more than one chelator
Two chelators can be used either sequentially (on different days) or in combination (both given on the same day) and, theoretically, all 3 currently available drugs could be used together, although this approach has not been tested. Dual-chelator therapy may improve compliance, improve organ-specific iron removal, minimize/reduce toxicity, and enhance iron removal if an additive or synergistic effect occurs.
Deferiprone with deferoxamine has been used since the 1990s at a variety of dose levels and schedules. Some protocols use sequential administration of the drugs, whereas others coadminister the 2 drugs on the same day, generally with deferiprone given in doses of 50-100 mg/kg/d (in 3 divided doses) daily and deferoxamine in doses of 20-50 mg/kg/d either subcutaneously or intravenously administered at least 2 days a week. A shuttling hypothesis whereby deferiprone binds iron and then redistributes it to deferoxamine has been proposed,46,47 and coadministration of these 2 drugs, which leads to an additive effect, may be optimal.
The combination of deferiprone and deferoxamine is currently the most effective means of reducing cardiac iron loading and should be initiated, if practical, for patients with significant cardiac siderosis. The superiority of this combination compared with deferoxamine alone was demonstrated in a randomized, placebo-controlled trial of 65 patients with mild to moderate cardiac iron loading (cardiac T2* = 8-20 ms).48 After a 12-month treatment period, subjects receiving combination therapy had significantly greater improvement in cardiac T2* (from 11.7 to 17.7 ms vs 12.4 to 15.7 ms, respectively) and in left ventricular ejection fraction (2.6% vs 0.6%, respectively) than those receiving deferoxamine with placebo. Furthermore, in single-arm trial of 15 patients with severe myocardial siderosis (T2* < 8 ms) and myocardial dysfunction, treatment with deferoxamine (mean dose, 38 ± 10.2 mg/kg for 5.3 days/week) combined with deferiprone (73.9 ± 4 mg/kg/d) was effective, resulting in a significant improvement in cardiac T2* (5.7 to 7.9 ms) and left ventricular ejection fraction (51.2% to 65.6%), as well as a reduction in serum ferritin and liver iron concentration after 12 months of treatment.49 Retrospective analysis of the efficacy of various chelation regimens confirmed that combination therapy with deferoxamine and deferiprone was superior with regard to cardiac iron removal.23
The combination of deferasirox and deferoxamine has not been extensively studied. In a pilot study, 14 patients with thalassemia major and liver iron concentration > 15 mg/g dry wt or > 5 mg/g dry wt plus evidence of iron-related organ dysfunction were treated with deferasirox (20-30 mg/k/d) daily and deferoxamine (35-50 mg/kg/d) for 3-7 days per week (longer durations for higher liver iron concentrations).50 Liver iron concentration significantly improved at a median follow-up of 29 weeks, and no excessive toxicity was seen. However, combination treatment with deferasirox and deferoxamine did not show additive (or synergistic) iron excretion in an iron-overloaded gerbil model, suggesting that the chelators may simply compete for a common iron pool.51 It is unclear if this will hold true in humans, but sequential rather than combination therapy would be expected to be a better strategy if the 2 chelators compete for the same iron pool. Such treatment could limit toxicity associated with each chelator and improve adherence, given that each chelator would be taken for fewer days. Further research regarding the safety and efficacy of treatment with deferasirox and deferoxamine is needed before recommendations for routine clinical practice can be made. Similarly, the combination of deferiprone and deferasirox, solely oral therapy, is particularly appealing, but this approach still needs to be studied.
Future research strategies
Most prior studies of chelator efficacy have consisted of retrospective analyses, small case series, and single-arm treatment trials. Randomized trials, when available, often lack the ability to compare changes in organ-specific iron loading with different dosing strategies, to assess the proportion of subjects who respond, and to stratify responses based on iron burden and transfusional iron intake. Deferoxamine, the first available chelator, was approved for clinical use before the routine practice of performing phase 3 studies. Prospective, randomized trials of deferiprone monotherapy compared with deferoxamine are limited, with many assessing cardiac iron removal in moderate cardiac siderosis. A large randomized trial of deferasirox and deferoxamine was conducted, but that study did not assess changes in cardiac iron and did not assess doses of deferasirox in excess of 30 mg/kg/d (an inadequate dose in a subset of patients). Furthermore, direct comparisons of the efficacy of the 2 oral chelators have not been performed, nor have studies been conducted comparing deferoxamine in combination with deferiprone or deferasirox. Moving forward, such randomized trials will be difficult, if not impossible, to conduct for a variety of reasons, including cost, patient numbers, and challenges in recruiting subjects willing to accept randomization if there is a parenteral treatment arm. Some of these obstacles and limitations may be overcome with comparative effectiveness trials, which can use large patient databases to assess the effectiveness of treatments in the “real world” setting as well as to compare costs and other factors.
Clearly, limitations to all 3 chelators exist, and a need for additional chelator options exists. One drug, FBS0701, a desazdesferrithiocin tridentate chelator modified to limit nephrotoxicity, has undergone phase 1 study.52 In 16 adults with transfusional iron overload, FBS0701 was administered daily for 1 week, with limited toxicity (eg, gastrointestinal problems and headache) noted. This drug is currently being investigated in phase 2 studies.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: Use of deferiprone, an agent that is not FDA approved, and information about the use of chelators in combination, which has not been studied.
Correspondence
Janet L. Kwiatkowski, MD, MSCE, Children's Hospital of Philadelphia, Division of Hematology, 3501 Civic Center Blvd, Colket Bldg, Room 11024, Philadelphia, PA 19104; Phone: (215) 590-3437; Fax: (215) 590-3992; e-mail: kwiatkowski@email.chop.edu.
