Abstract
Approximately 12 000 adults are diagnosed with acute myeloid leukemia (AML) in the United States annually, the majority of whom die from their disease. The mainstay of initial treatment, cytosine arabinoside (ara-C) combined with an anthracycline, was developed nearly 40 years ago and remains the worldwide standard of care. Advances in genomics technologies have identified AML as a genetically heterogeneous disease, and many patients can now be categorized into clinicopathologic subgroups on the basis of their underlying molecular genetic defects. It is hoped that enhanced specificity of diagnostic classification will result in more effective application of targeted agents and the ability to create individualized treatment strategies. This review describes the current treatment standards for induction, consolidation, and stem cell transplantation; special considerations in the management of older AML patients; novel agents; emerging data on the detection and management of minimal residual disease (MRD); and strategies to improve the design and implementation of AML clinical trials.
Introduction
Approximately 12 000 adults are diagnosed with acute myeloid leukemia (AML) in the United States annually, with a median age of 67 years.1 Despite advances in therapeutics and supportive care, the majority of patients with AML die from their disease. Acute promyelocytic leukemia, which comprises ∼ 10% of adults with AML, is an important exception to this general statement. In this subtype, not considered in detail here, > 75% of patients are cured with a combination of anthracycline-based chemotherapy, all-trans retinoic acid, and arsenic trioxide. For some APL patients, it is possible to eliminate cytotoxic chemotherapy altogether and to achieve cure with arsenic and all-trans retinoic acid alone. For all other subtypes of AML, the mainstay of initial treatment was developed nearly 40 years ago as a combination of cytosine arabinoside (ara-C) with an anthracycline, and this regimen remains the worldwide standard of care. Approximately 70%-80% of patients < 60 years of age will achieve complete remission,2,3 but most ultimately relapse and overall survival is only 40%-45% at 5 years. Among patients > 60 years of age, 40%-50% of those with a good performance status can achieve complete remission, but cure rates are < 10% and median survival is < 1 year.4,5 The outlook for older AML patients has not changed in 3 decades, and is even worse for older patients with unfavorable cytogenetics and/or poor performance status.
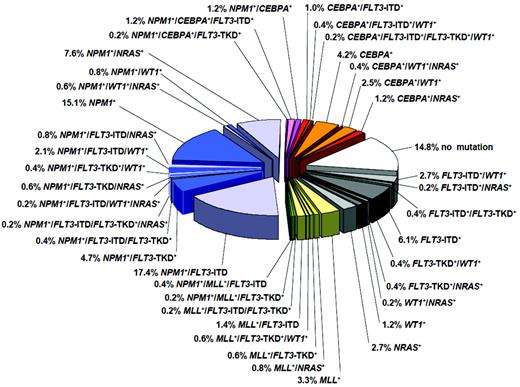
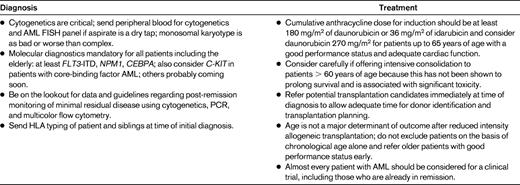
In the last few years, there have been several practice-changing developments in the diagnosis and treatment of AML. The old “favorable, intermediate, and unfavorable” prognostic categories, which were based on cytogenetic risk groups, are no longer adequate. Advances in genomics technologies have identified AML as a genetically highly heterogeneous disease, and an increasing number of AML patients can now be categorized into distinct clinicopathologic subgroups on the basis of their underlying molecular genetic defects. Cytogenetically normal patients, who comprise the largest subgroup and have historically been assigned an “intermediate” prognosis, can now be further divided into a myriad of molecular subgroups, some of which are known to have significant prognostic implications (Figure 1). For example, mutations in FLT3-ITD (FML-like tyrosine kinase 3 internal tandem duplication) have been associated with an aggressive disease phenotype and poor outcomes. In contrast, patients with biallelic mutations in CEBPA (CCAAT enhancer-binding protein α) and NPM1 (nucleophosmin 1) without concomitant mutations in FLT3-ITD have significantly more favorable outcomes. Prognostically important mutations have also been identified in patients with abnormal cytogenetics; for example, mutations in KIT may negate the “favorable” classification previously associated with t(8;21).6 Although only FLT3-ITD, NPM1, and biallelic CEBPA mutations have been incorporated into AML clinical practice guidelines so far, many more are likely to become standard assessments in the near future. Table 1 provides notes for the clinician on the standard of care for AML. Novel molecular markers and targets are emerging with such startling rapidity that one hopes the needle might finally move forward with a jump, rather than a wiggle, in this devastating disease.
Making old drugs new again
The treatment paradigm for AML generally includes remission induction, followed by consolidation with either 1-4 cycles of chemotherapy or stem cell transplantation. The drugs for remission and consolidation have, for the most part, been variations on a theme of ara-C combined with an anthracycline or anthracenedione. Yates et al first reported the results of a pilot trial of infusional cytarabine combined with daunorubicin in AML in 1973.7 The treatment was called “7&3 DNR 45” to indicate the dose of daunorubicin, 45 mg/m2. In 1981, the Cancer and Leukemia Group B (CALGB) published a randomized trial demonstrating the superiority of daunorubicin 45 mg/m2 versus daunorubicin and Adriamycin 30 mg/m2.8 In the 1990s, both idarubicin 12 mg/m2 and mitoxantrone 12 mg/m2 were established as reasonable substitutes for daunorubicin, and a large meta-analysis comparing trials of idarubicin 12 mg/m2 with daunorubicin 45-50 mg/m2 showed somewhat superior rates of complete remission (62% vs 53%, P = .002) and overall survival (13% vs 9%) with idarubicin.9 Trials from the Southwest Oncology Group, CALGB, and the Acute Leukemia French Association (ALFA) have shown high rates of complete remission with acceptable toxicity for intensified doses of daunorubicin 70-95 mg/m2 for 3 days.10–12 In a large randomized trial, the Eastern Cooperative Oncology Group (ECOG) demonstrated that doubling the daunorubicin dose from 45 to 90 mg/m2 for 3 days (cumulative dose, 270 mg/m2) resulted in both higher rates of complete remission (7.6% vs 57.3%, P < .001) and improved overall survival (23.7 vs 15.7 months, P = .0003) in patients with AML under the age of 60 years.2 There was no benefit with the higher dose in patients older than 50 years or in those with unfavorable cytogenetics. Lowenberg et al also found a survival benefit with intensified daunorubicin dose in older patients between 60 and 65 years of age (38% vs 23%).13 However, a randomized trial of > 1000 patients < 65 years of age from the Japan Acute Leukemia Study Group (JALSG) failed to show any benefit of intensified daunorubicin (cumulative dose, 250 mg/m2) over standard-dose idarubicin (cumulative dose, 36 mg/m2).14 Importantly, none of these studies identified excess toxicity of any kind, including cardiac, with increased doses of daunorubicin. It can be concluded that dose intensification of daunorubicin to 90 mg/m2 for 3 days during induction should be strongly considered for AML patients < 65 years of age with a good performance status and adequate baseline cardiac function.
The optimal dose of ara-C during AML induction and consolidation is also controversial. Neither doubling the dose of ara-C from 100 mg/m2 to 200 mg/m215 nor prolonging the infusion from 7 to 10 days16 resulted in additional benefit. Randomized trials of infusional ara-C 200 mg/m2 for 7 days versus 500 mg/m2 every 12 hours for 12 doses or 2 g/m2 every 12 hours for 12 doses did not result in improved overall survival, but a meta-analysis demonstrated the superiority of high-dose ara-C during induction, specifically with respect to long-term survival in patients < 60 years of age.17 Several institutions, including the M. D. Anderson Cancer Center, routinely use high-dose ara-C (defined as ≥ 1 g/m2 per dose) during induction,18 and the National Comprehensive Cancer Network (NCCN) lists high-dose ara-C 2-3 g/m2 every 12 hours for 3 days in combination with daunorubicin or idarubicin as an alternative induction strategy, but recommends caution using this regimen outside of a clinical trial.19 Recent data from the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology and the Swiss Group for Clinical Cancer Research (HOVON-SAKK) Collaborative Study Group suggest no benefit of escalation of ara-C above 1000 mg/m2 during induction.20 However, it is important to note that patients on this trial were treated with a double-induction strategy in which the first cycle included idarubicin combined with either ara-C 200 mg/m2 for 6 days or 1000 mg/m2 every 12 hours for 5 days and the second cycle included amsacrine combined with either ara-C 1000 mg/m2 every 12 hours for 6 days or ara-C 2000 mg/m2 every 12 hours on days 1, 2, 4, and 6. Patients in remission then received either a chemotherapy consolidation cycle or autologous or allogeneic stem cell transplantation according to a risk-adapted design.20 Higher doses of ara-C (18 g/m2/cycle) have been standard for consolidation therapy in younger patients since the early 1990s21 and are associated with a survival benefit in core-binding factor AML.22–24 More recently, this high-dose ara-C consolidation approach has also been recommended for patients with mutations in NPM1 and CEBPA in the absence of FLT3-ITD mutations.25 It is unclear whether similar results can be obtained using intermediate doses of ara-C in these cytogenetic and molecular subgroups. A recent analysis of 933 patients 15-60 years of age treated in Germany between 1996 and 2003 compared consolidation with mitoxantrone combined with total doses of ara-C 12 g/m2 versus 36 mg/m2.26 All patients were initially treated with a double induction including ara-C 10 g/m2, which resulted in cumulative doses of 32 g/m2 or 56 g/m2 throughout the treatment program. There were no significant differences in survival by intention-to-treat or as-treated analyses, and there were no differential effects according to age or molecular/cytogenetic risk groups.26 Prospective, randomized trials are still needed to clarify the timing, schedule, and total dose of ara-C for younger patients with AML not undergoing allogeneic transplantation.
Other purine nucleoside analogs such as clofarabine, cladribine, and fludarabine have been used instead of and in addition to ara-C in a variety of induction regimens. Each has different pharmacokinetic and toxicity profiles and all are active in AML, but to date, none has proven to be superior to ara-C in AML induction or consolidation. Cladribine has been added to daunorubicin 60 mg/m2/d for days 1-3 and cytarabine 200 mg/m2/d for days 1-7 in a randomized trial compared with daunorubicin/cytarabine alone in patients < 60 years of age, and showed acceptable toxicity, improved rate of complete remission after one induction cycle, and a possible survival benefit.27
Older AML patients: when to lower the intensity
Age ≥ 60 years has consistently been identified as an independent adverse prognostic factor in AML, and there are very few long-term survivors in this age group.5 Poor outcomes in elderly AML patients have been attributed to both host- and disease-related factors, including medical comorbidities, physical frailty, increased incidence of antecedent myelodysplastic syndrome and myeloproliferative disorders, and higher frequency of adverse cytogenetics.28 Older patients with multiple poor-risk factors have a high probability of early death and little chance of long-term disease-free survival with standard chemotherapy. In a retrospective analysis of 998 older patients treated with intensive induction at the M.D. Anderson Cancer Center, multivariate analysis identified age ≥ 75 years, unfavorable karyotype, poor performance status, creatinine > 1.3 mg/dL, duration of antecedent hematologic disorder > 6 months, and treatment outside a laminar airflow room as adverse prognostic indicators.29 Patients with 3 or more of these factors had expected complete remission rates of < 20%, 8-week mortality > 50%, and 1-year survival < 10%. The Medical Research Council (MRC) identified cytogenetics, WBC count at diagnosis, age, and de novo versus secondary disease as critical factors influencing survival in > 2000 older patients with AML, but cautioned in their conclusions that less objective factors, such as clinical assessment of “fitness” for chemotherapy, may be equally important in making treatment decisions in this patient population.30 It is hoped that data from comprehensive geriatric assessments of functional status, cognition, mood, quality of life, and other measures obtained during ongoing cooperative group trials will improve our ability to predict how older patients will tolerate treatment.
Despite the grim statistics, it is clear that selected older patients with AML will benefit from intensive therapy, and it is a critical challenge for clinicians to quickly and accurately identify these individuals. For example, patients without adverse risk factors using the M.D. Anderson criteria have an expected complete remission rate of > 60%, induction mortality of 10%, and a 1-year survival rate of > 50%.29 Registry data from nearly 3000 unselected older patients in Sweden showed improved rates of early death and long-term survival in those treated with intensive therapy versus palliation.31 Older patients with core-binding factor leukemias or NPM1 mutations have complete remission rates of > 80% and improved overall survival with intensive chemotherapy induction.6,32 Patients > 60 years of age, like younger patients, should be treated with the goal of achieving remission: complete remission is correlated with survival and quality of life after chemotherapy and also leads to better outcomes after allogeneic transplantation with reduced-intensity conditioning.33 Standard chemotherapy using 3 days of 60 mg/m2 of daunorubicin or 12 mg/m2 of idarubicin with 100 mg/m2 of cytarabine for 7 days still carries the highest probability of complete remission and should be considered in older patients without multiple adverse-risk features who do not have the option of participating in a clinical trial. Escalation of the daunorubicin dose to 90 mg/m2 for 3 days can be considered for patients ≤ 65 years of age.13
Numerous efforts have been made to preserve efficacy while reducing toxicity in older patients with AML. The prototype for low-intensity induction over the last several decades has been low-dose ara-C, which resulted in a complete remission rate of 18% and improved overall survival when randomized to supportive care and hydrea.34 Low-dose ara-C is almost never effective in patients with unfavorable cytogenetics. The hypomethylating agent azacitidine has been studied in a randomized trial versus best supportive care, low-dose ara-C, or intensive chemotherapy in patients with high-risk myelodysplastic syndrome.35 A subset analysis of older patients who met the World Health Organization criteria for AML with 20%-30% BM blasts showed 18% complete remission, with a survival benefit in favor of azacitidine (24.5 vs 16 months, P = .005), including higher 2-year survival (38% vs 0%, P = .01) in patients with adverse cytogenetics.36 The quality of life and economic implications of improved survival with versus without complete remission in AML are not known. The hypomethylating agent decitabine has been given in 5- and 10-day schedules to older patients with AML, with 24% and 47% rates of complete remission, respectively.37,38 The drug was well tolerated and complete responses were seen in patients with adverse prognostic features. Induction with 10 days of decitabine is being tested in a new CALGB trial of older AML patients, and may prove a worthy rival to conventional induction if the encouraging phase 2 data are reproduced. Monotherapy with clofarabine has been tested in US and European trials, resulting in 38% and 32% rates of complete remission, respectively, in older patients with adverse prognostic features.39,40 An ECOG randomized trial of clofarabine versus daunorubicin (60 mg/m2 for 3 days) and cytarabine in patients > 60 years of age is ongoing.
There are no prospective, randomized trials confirming the benefit of postremission chemotherapy in older patients. Consolidation with high-dose ara-C produces significant toxicity in patients > 60 years of age and is discouraged. A randomized trial by ALFA in patients ≥ 65 years of age in first remission compared consolidation with 6 monthly outpatient courses of an anthracycline combined with 60 mg/m2 ara-C administered subcutaneously every 12 hours for 5 days with a single cycle of an anthracycline combined with 7 days of ara-C 200 mg/m2 as a continuous infusion.41 Disease-free and overall survival were improved with the longer, ambulatory treatment, as were duration of rehospitalization and transfusion requirements. In contrast, another randomized trial in Europe found that a second, intensive consolidation cycle was better than prolonged, low-dose oral therapy in older patients who had already received one postremission consolidation treatment.42 Postremission maintenance therapy with low-dose ara-C and long-term, low-dose multi-agent chemotherapy have been shown to improve disease-free survival but not overall survival.43,44 The anti-CD33 immunotoxin conjugate gemtuzumab ozogamicin was compared with no postremission therapy in older patients and showed no benefit.45 Finally, allogeneic stem cell transplantation with reduced-intensity conditioning is increasingly feasible for patients well into their 70s, and registry data suggest that age is not the major predictor of transplantation outcome in these patients.32
Novel and targeted agents
Several agents with initially promising results have failed to improve outcomes in randomized trials, including the P-glycoprotein modulator zosuquidar, the sulfonylhydrazine alkylator laromustine, and gemtuzumab ozogamicin. Although gemtuzumab ozogamicin was withdrawn from the US market, it may be of benefit for patients with favorable cytogenetics or acute promyelocytic leukemia. Efforts are also under way to improve and optimize ara-C, for example, via conjugation to the lipid moiety elaidic acid, which allows bypassing of the nucleoside transporter protein hENT1 (Elacytarabine; Clavis Pharma). Another novel formulation, CPX-351 (Celator Pharmaceuticals), fixes a 5:1 ratio of cytarabine to daunorubicin within a liposomal carrier. Novel topoisomerase II inhibitors are also under development (eg, Vosaroxin; Sunesis Pharmaceuticals).
The concept of targeting leukemic cells and sparing normal ones from the broad attack of chemotherapy is appealing but problematic, because there are many potential targets in AML cells and they may require simultaneous targeting. Mutations in FLT3-ITD are identified in 20% of all AML patients and in 28%-34% of those with normal cytogenetics. These mutations are associated with a poor prognosis, especially when there is a high mutant to wild-type allelic ratio. The oral FLT3 inhibitor midostaurin has shown biologic activity as a single agent in reducing blasts in patients with relapsed FLT3 mutated AML46 and it is currently being combined with induction chemotherapy in a randomized trial for patients < 60 years of age. Sorafenib, a Raf-1 kinase, VEGF, and FLT3 inhibitor, has been safely combined with induction chemotherapy47 and is being combined with standard induction in a CALGB trial of older AML patients with mutations in FLT3-ITD. It has also been used successfully as monotherapy before and after allogeneic stem cell transplantation in selected patients with FLT3-mutated AML.48 Finally, AC220, a more potent and selective next-generation FLT3 inhibitor, is showing promising activity in clinical trials and others are under development.49
The farnesyltransferase inhibitor tipifarnib failed to win approval as a single-agent for treatment of older patients with AML due to inadequate efficacy, but the RASGRP1/APTX gene-expression ratio has been found to predict response to tipifarnib, and further study in selected patients using this 2-gene classifier is underway.50 The dual SRC/ABL kinase inhibitor dasatinib is being tested in AML patients with mutations in KIT. Bortezomib, an inhibitor of NF-κB, has been safely combined with idarubicin and cytarabine51 and is currently being evaluated in combination with decitabine versus decitabine alone in newly diagnosed AML patients > 60 years of age. Efforts to target leukemia stem cells and the BM microenvironment are discussed below.
Extending the borders of allogeneic stem cell transplantation
Allogeneic stem cell transplantation is probably still the most effective anti-AML therapy. As the donor pool expands to include more unrelated donors and umbilical cords, and as treatment-associated morbidity and mortality rates decline with improvements in HLA matching, antimicrobial therapy, and management of GVHD, it is becoming increasingly feasible for more patients with AML to undergo the procedure. It is critical that the transplantation workup be initiated at the time of initial diagnosis to allow adequate time for donor identification and other preparations. All AML patients with complex cytogenetics or monosomal karyotype who have a good performance status should be considered for allogeneic transplantation. Pilot data suggest that proceeding with transplantation early in these patients, regardless of the outcome of induction, may be of benefit.52 Patients should be considered for transplantation regardless of chronological age (the literature reports an 82-year-old cord blood recipient!),53 but it is acknowledged that posttransplantation quality of life data are very limited for patients > 60 years of age. Patients with FLT3-mutated AML and cytogenetically normal, “triple-negative” (FLT3, CEBPA, and NPM1 unmutated) disease may be considered for allogeneic transplantation, preferably as part of a clinical trial because the benefits of the procedure in these settings have not been firmly established.
Eradicating MRD
The current definition of complete remission in AML, which includes achievement of specific hematologic parameters and < 5% BM myeloblasts by morphology, is inadequate and results in a false sense of security for patients and practitioners. It has been clearly demonstrated that persistent cytogenetic and/or molecular abnormalities after induction chemotherapy are poor independent prognostic indicators in AML.54,55 For patients without baseline cytogenetic or molecular abnormalities, demonstration of minimal residual disease (MRD) by multicolor flow cytometry is also correlated with inferior outcomes.56 Postremission assessments of MRD using PCR and sensitive flow cytometry techniques are rapidly becoming part of the standard of care in AML, as they already are in acute promyelocytic leukemia and acute lymphoid leukemia.57 However, the optimal timing and frequency of such assessments are not clear, nor is their application in clinical care. For example, should positive assessments be repeated over a period of time before recommending intervention? Should all patients with positive MRD be referred for allogeneic stem cell transplantation or chemotherapy salvage or a clinical trial? Clinical trials using MRD as a guide for therapy have shown improved outcomes in childhood AML, and similar trials are under development for adults.58
Although technologic advances are making real-time identification of MRD more feasible and readily available, the subject of MRD and its clinical and scientific significance has been in the literature for decades. The concept that leukemic cells are “left over” after treatment and eventually proliferate and cause disease relapse is intuitive and supported by laboratory data. A more difficult question, however, is whether these cells are, or arise from, so-called leukemia stem cells (LSCs). Whereas the exact characteristics of LSCs remain controversial, most agree that they are defined by their ability to recapitulate disease when transplanted into immunodeficient mice.59 Data suggest that AML is composed of biologically distinct leukemic stem, progenitor, and blast populations in which the LSCs comprise 0.1%-1% of the blasts and are largely quiescent but capable of endless self-renewal60 (Figure 2). A high frequency of LSCs at diagnosis is associated with a poor outcome in AML61 and, similarly, high expression of an LSC gene signature has also been independently associated with inferior survival.62 Isolation of putative LSCs is becoming more feasible, but remains extremely challenging in the posttreatment setting, when their population is tiny and, presumably, well-hidden within the BM niche. LSCs have distinct characteristics, including aberrant surface immunophenotype; dysregulated programs for survival, apoptosis, and differentiation; and complex interactions with their surrounding BM microenvironment, the LSC niche.60 All of these factors contribute to the incredible resilience of LSCs in surviving the harshest of antileukemic therapies, including allogeneic stem cell transplantation. Ara-C, which is arguably still the most active antileukemic drug, is notably ineffective against LSCs.63 There are ongoing clinical and laboratory efforts to identify agents that specifically target LSCs by exploiting their unique biologic features. Although there is overlap between the surface immunophenotype of LSCs and normal hematopoietic stem cells, antibody-based therapies are under development against antigens believed to be preferentially expressed by LSCs (eg, CD123, CD44, CD47, and CLL-1). Drugs that target the PI3K pathway and/or the transcription factor complex NF-κB may be especially active against LSCs. Finally, it may be possible to attack LSCs by targeting their interactions within the BM microenvironment, for example by disruption of regulatory pathways (Wnt, Notch, HOX, etc) or interruption of homing signals between CXC chemokine receptor-4 (CXCR4) and stromal cell-derived factor-1 (SCF-1, CXCL12).60 Ideally, LSC-targeted agents will be used during both the remission-induction and the postremission periods, probably in conjunction with conventional chemotherapy.
Accelerating drug development: making clinical trials faster and better
A recurrent theme in most of oncology is the observation that it seems to take forever, or at least decades, for new drugs or treatment regimens to arrive in the clinic. Several current strategies may facilitate this process in AML.
1. More development of existing drugs in addition to searching for new ones
Whereas the search for a “magic bullet” is irresistible, the heterogeneous pathogenesis and molecular genetics of AML suggest that combination therapy may remain superior to any single agent, and that tailored, personalized treatment based on the specific biologic features of the leukemic cells should be the goal. In pediatric acute lymphoblastic leukemia, major improvements in outcomes have been achieved without the addition of novel agents, but rather via optimizing the combination, schedule, and duration of treatment using existing (and decades-old) drugs. One intriguing proposal is to use the gene-expression signature generated from drugs that effectively ablate LSCs (eg, parthenolide) to search publicly available databases for other similar signatures.64 This may allow existing compounds with well-characterized clinical profiles and, in the case of off-patent compounds, easy availability, to be quickly introduced into AML regimens.
2. Increase accrual to clinical trials
Fewer than 5% of adults with cancer in the United States participate in clinical trials, in contrast to pediatric cancer patients, 60% of whom are enrolled in trials.65 Accrual is also higher in Europe. In a recent French survey, 25% of AML patients among 1066 adults with acute leukemia were enrolled in clinical trials.66 There is significant momentum to enhance physician and patient education about clinical trials and to increase collaboration between academic centers and cooperative groups.
3. Change the paradigm for drug development
Increasing participation in clinical trials is urgent in AML because there is a growing realization that the traditional phase 1-3 drug-development paradigm is ineffective in this disease.67 Phase 1 trials generally offer novel agents to patients with relapsed and refractory disease. Noncytotoxic, molecularly targeted agents do not have much chance for success in this setting, and signals of their true biologic efficacy may be missed. Many scientifically interesting and potentially useful agents seem to die early in development because they fail to demonstrate a signal of efficacy in the phase 1 setting. At least some of these agents should be considered instead in trials to prolong response duration and survival in AML patients who have already achieved remission. Phase 2 trials in AML are often small and may give misleading efficacy signals. Given the heterogeneity of the disease, subgroup analyses based at least on age, performance status, cytogenetics, and molecular features are essential, yet these are meaningless when the total cohort includes only 20-50 patients. There is increasing support for randomized phase 2 trials, including adaptive randomization and “pick-the-winner” strategies designed to quickly compare new treatments with existing standards using as few patients as possible and to proceed only with those that meet preset efficacy benchmarks. Finally, phase 3 trials in AML are often unbearably slow and expensive to complete and, unfortunately, for the most part have resulted in only small improvements in outcome. In addition, as the number of molecularly and clinically defined subgroups increases, it has become more and more difficult to determine a reasonable control arm for new phase 3 trials. This is especially true for older patients, for whom anything from hospice to stem cell transplantation might be a reasonable option. It is hoped that further refinements in the molecular characterization of AML will allow the identification of more homogeneous treatment groups and tailored therapeutics.
Disclosures
Conflict-of-interest disclosure: The author has consulted for Celgene, ChemGenex, EpiCept, and Boehringer Ingelheim. Off-label drug use: None disclosed.
Correspondence
Gail J. Roboz, Weill Medical College of Cornell University, 179 East 70th St, Apt 7B, New York, NY 10021; Phone: (212) 746-3207; Fax: (212) 746-8246; e-mail: gar2001@med.cornell.edu.