Abstract
An 18-year-old man has severe hemophilia A that has been complicated by a high-titer inhibitory antibody (peak 170 BU/mL). He had previously failed a trial of immune tolerance induction (ITI) using daily high-dose (100 units/kg/d) factor VIII (FVIII) for 20 months and would like to know if immunomodulatory agents, with or without another course of ITI, might eradicate the inhibitor.
Introduction
To review the current best evidence of the effect of immunomodulatory agents in hemophilia A patients with inhibitory antibodies who previously have failed to respond durably to immune tolerance induction (ITI), we performed a comprehensive review of the published literature indexed on the OVID Medline database using the search terms hemophilia A (MESH, no restrictions, 15 755 hits) AND immune tolerance (MESH, no restrictions, 27 844 hits) AND immunosuppression (MESH, immunosuppressive agents, 87 696 hits) OR rituximab (MESH, including anti-CD20 and Rituxan, 7185 hits) OR cyclophosphamide (MESH, no restrictions, 40 079 hits) OR steroids, prednisone, hydrocortisone, corticosteroids, dexamethasone (MESH, no restrictions, 26 059, 31 640, 57 045, 30 008, 40 273 respective hits) OR IVIg (MESH, no restrictions, 8155 hits) between 1948 and the first week of June 2011. The MESH terms rapamycin OR bortezomib (including Velcade and proteasome inhibitor) OR azathioprine OR vincristine OR cyclosporine OR mycophenolate yielded no additional articles. This search identified 51 articles, and an additional 5 articles were identified from the reference lists of 3 of the articles. Forty-fix papers were excluded; 15 described initial efforts at eradication of the inhibitor only, 14 were review articles, 7 were preclinical studies, 5 dealt exclusively with acquired hemophilia, 3 involved no immune suppression, 1 dealt only with mild/moderate hemophilia, and 1 was a duplicate. Of the remaining 10 studies,1–10 there were no controlled trials and the majority of studies were case reports or small case series on the use of immunomodulatory agents following failure of prior ITI (Table 1).
Summary of published cases using immunomodulatory agents for suppression/eradication of refractory inhibitors in hemophilia
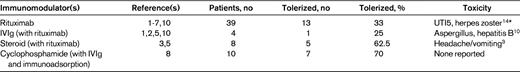
*Aleem et al. used rituximab as a single-agent first-line therapy.
The Malmö protocol, consisting of high-dose factor VIII (FVIII) daily with IVIg 2.5-5 g on day 1 followed by 400 mg/kg on days 4-9, cyclophosphamide 12-15 mg/kg IV days 1 and 2 and 2-3 mg/kg orally on days 3-12, and Sepharose A immunoadsorption for inhibitors with titers > 10 BU/mL,8 was first described in the context of rescue of several patients who previously had failed to respond to more limited regimens of ITI incorporating FVIII infusions.8 Subsequent reports,11–13 however, concern its use as a regimen for initial eradication of the inhibitor, and no recent data for patients with long-standing inhibitory antibodies that have proven refractory to protracted infusions of FVIII alone are available.
More recently, a systematic review of the use of rituximab in patients with hemophilia and inhibitors (including some who had previously failed to respond to ITI using FVIII infusions) identified 44 published cases, with patients ranging in age from 1-70 years, who received 2-11 weekly doses of the agent (375 mg/m2). Of these, 55% achieved durable tolerance and patients who received rituximab concomitantly with FVIII infusions or other immunomodulatory agents were more likely to achieve immune tolerance than those who did not.5 Patients with severe hemophilia, however, were less likely to achieve durable tolerance than those with mild or moderate disease.5 Twenty-two cases had previously failed ITI1,2,4–7 ; of these, 5 received concomitant corticosteroids, 2 IVIg, 1 vincristine, and 1 cyclosporine A. Fifteen of the 22 patients achieved negative inhibitor titers, but only 10 had durable long-term responses.
Collins et al reported 15 consecutive cases of the use of rituximab in severe hemophilia A patients from the United Kingdom, ranging in age from 2-37 years, all of whom had previously failed at least one prior regimen of ITI.3 Four weekly doses of 375 mg/m2 were administered. Six of 15 patients achieved negative inhibitor titers, but only 2 achieved durable tolerance, and 0 of the 3 patients receiving rituximab without concurrent factor VIII infusions achieved negative inhibitor titers. In a separate report, 2 adolescent patients who had previously failed to respond to standard ITI received IVIg and cyclosporine A in addition to rituximab,10 and both achieved negative antibody titers (1 recurred). In most reports, rituximab appeared to be well tolerated, although infections (including pathogens such as hepatitis B, varicella, or aspergillus) have occurred, in some cases in association with additional immunomodulation.10,14
In conclusion, limited evidence suggests that immunomodulatory agents may lead to the suppression or eradication of inhibitory antibodies in selected individuals with severe hemophilia A who previously have failed ITI. If administered alongside daily infusions of FVIII, either rituximab (insufficient data to recommend a regimen or additional immunomodulation) or cyclophosphamide with IVIg and immunoadsorption (if titer > 10 BU/mL) (Malmö protocol) may be considered (grade 2C). The use of immunomodulatory agents may be associated with an increased risk of infection.
Disclosures
Conflict-of-interest disclosures: M.U.C. declares no competing financial interests. P.F.F. has received research funding from NovoNordisk, Bayer Healthcare, Baxter Healthcare, Biogen IDEC, and PTC Biotherapeutics and has received honoraria from NovoNordisk, Baxter Healthcare, and Bayer Healthcare. Off-label drug use: Rituximab, cyclosporine, tacrolimus (immunosuppression), immunomodulatory agents used off-label in hemophilia immune tolerance.
Correspondence
Michael U. Callaghan, Department of Pediatrics, Wayne State University School of Medicine, 2nd Floor Carls Bldg, 540 E Canfield, Detroit, MI 48201; Phone: (248) 953-5250; Fax: (313) 745-5237; e-mail: mcallagh@med.wayne.edu.
