Abstract
Extended-field and subtotal nodal radiation therapy (RT), developed in the 1960s, was the first reliably curative treatment for early-stage Hodgkin lymphoma (HL). However, the large volume of normal tissue irradiated resulted in significant delayed toxicity, including cardiac disease and second cancers (SCs). The 30-year cumulative incidence of heart disease among adult survivors receiving 40-45 Gy of extended-field or mantle RT is approximately 30%; the incidence of SCs is similar. Improving disease control while reducing the toxicity of treatment has been a major objective of HL trials for more than 2 decades. Contemporary involved-field RT (IFRT) reduces irradiated volumes and produces significant reductions in normal tissue dose compared with historic treatments. Recent data indicate that, compared with mantle RT, IFRT reduces the relative risk of breast cancer among young females receiving mediastinal RT by approximately 60% and also reduces cardiac dose. The recent transition to involved-node RT allows further reductions in normal tissue dose. Response-adapted therapy is being evaluated in clinical trials as a means of identifying those patients most likely to benefit from treatment reduction or intensification, enhanced screening will facilitate early intervention to reduce the clinical burden of late effects, and there is increasing interest in elucidating the genetic correlates of treatment toxicity.
Introduction
In 1962, Hodgkin lymphoma (HL) was as lethal as lung cancer is today. That year, groundbreaking work describing extended-field radiation therapy (RT) as a curative treatment for HL was published by Kaplan, and the 5-year survival for localized disease increased to more than 70%. However, it eventually became apparent that these large RT volumes were associated with significant delayed toxicities among long-term survivors, including second cancers (SCs), heart disease, and endocrine dysfunction. Since that time, improvements in RT delivery and an evolving understanding of how to use RT in conjunction with modern chemotherapy are expected to result in substantial reductions in the incidence of late effects. This review outlines some of the relevant changes in RT for HL and how they affect normal tissue exposure and late toxicity, particularly SCs and heart disease.
Expressing risk using clinically meaningful measures
There are different ways of expressing risk that are not equally useful for understanding the clinical burden of late effects. The most commonly used descriptions of risk are the standardized incidence ratio (SIR), a ratio of the observed rate of the adverse event divided by the expected rate in the general population; the absolute excess risk (AER), typically expressed as the number of excess cases per 104 person-years of observation; and the cumulative incidence, the proportion of patients expected to develop the late effect by a specified follow-up time.
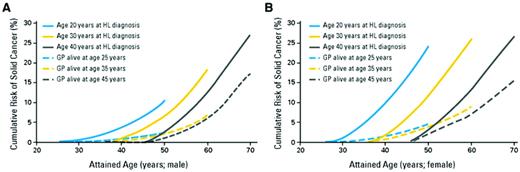
When a given diseases is rare in the general population (eg, leukemia and sarcoma), a small number of excess cases among survivors may produce a very high SIR, even though relatively few survivors are affected. Consequently, AER better reflects the clinical burden of a late effect in a cohort and is a better indicator of the extent to which a late effect produces excess morbidity or mortality. The cumulative incidence is arguably the most clinically interpretable expression of risk. Estimation of the cumulative incidence of delayed events requires appropriate statistical handling of competing causes of death (eg, from HL). One should be aware that actuarial estimates using the Kaplan-Meier method are not appropriate for estimating the cumulative incidence of late effects, because when there are significant risks of death (eg, among high-risk HL patients), actuarial methods will overestimate the true risk of the late effect. There are specific methods for handling competing risks that should be used to estimate the cumulative incidence of SCs and other late effects among HL survivors.1 Like all measures of risk, the cumulative incidence of most late effects is dependent on both age at exposure and attained age (Figure 1).
Cumulative incidence of solid cancers among 5-year survivors of HL compared with controls of the same age in the general population. (A) Males (n = 10 619 survivors). (B) Females (n = 8243 survivors). (From Hodgson et al.8 Reprinted with permission. Copyright 2007, American Society of Clinical Oncology. All rights reserved.)
Cumulative incidence of solid cancers among 5-year survivors of HL compared with controls of the same age in the general population. (A) Males (n = 10 619 survivors). (B) Females (n = 8243 survivors). (From Hodgson et al.8 Reprinted with permission. Copyright 2007, American Society of Clinical Oncology. All rights reserved.)
Late effects: historic treatment
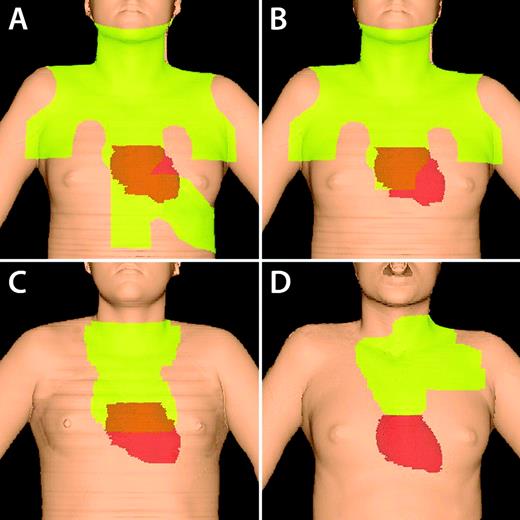
Most of the widely cited cohort studies of SC risk among HL survivors include a substantial proportion of patients whose sole primary treatment was extended-field RT.2–6 These RT fields typically encompassed the whole neck, bilateral axillae, the entire length of the mediastinum, the spleen, and para-aortic nodes (Figure 2). Patients were often prescribed higher doses than are currently in use (ie, 36-45 Gy) and were treated without customized lung shielding. In addition, patients were exposed to cumulative doses of alkylating agents no longer relevant to most primary treatment regimens. As noted above, although patients receiving these large RT fields were generally cured, significant delayed toxicity was observed among long-term survivors.
Changes in RT fields over time. Figures are 3-dimensional reconstructions of RT fields based on computed tomography imaging. Light green illustrates irradiated field. The true heart position is shown. (A) Mantle and upper abdomen (“spade”) field. Note the large volume of heart and left breast irradiated. (B) Mantle field. (C) IFRT for mediastinal disease without axillary disease. Less breast tissue is treated, although inclusion of the subcarinal nodes encompasses the proximal coronary arteries. (D) INRT for a patient with mediastinal, low neck, and high axillary disease. The patient was also treated with active breath hold to decrease heart dose.
Changes in RT fields over time. Figures are 3-dimensional reconstructions of RT fields based on computed tomography imaging. Light green illustrates irradiated field. The true heart position is shown. (A) Mantle and upper abdomen (“spade”) field. Note the large volume of heart and left breast irradiated. (B) Mantle field. (C) IFRT for mediastinal disease without axillary disease. Less breast tissue is treated, although inclusion of the subcarinal nodes encompasses the proximal coronary arteries. (D) INRT for a patient with mediastinal, low neck, and high axillary disease. The patient was also treated with active breath hold to decrease heart dose.
Second cancers
Studies of patients treated primarily before 1995 have reported that HL survivors experience in excess of approximately 45-80 malignancies per 104 person-years of follow-up.3,5,7 Most of these are solid tumors (30-60 cases per 104 person-years). Leukemia and non-HL each contribute ∼ 9 cases per 104 person-years. The cumulative incidence of SC among a cohort of 18, 862 5-year survivors treated between 1970 and 2001 is shown in Figure 1. It can be seen that the excess risk is higher among females than males and increases with time.
The risk of breast cancer following mantle RT has been of particular concern. Mantle RT (35-45 Gy to axillary, mediastinal, and neck nodes) is associated with a 2- to 20-fold increased relative risk of breast cancer, depending on the age at treatment.2,4,7–9 Ng et al reported a 20-year cumulative risk of SCs of 23% after mantle RT with para-aortic and spleen RT (median age at RT, 25 years; median prescribed dose, 40 Gy).9 Breast cancer accounted for almost 40% of SCs among female survivors. Other studies of survivors treated with similar RT fields and doses have reported a 30-year cumulative incidence of breast cancer of approximately 20%-30% among females treated before age 30.10–12 Most studies have found that the AER of breast cancer is highest among females treated before age 203,7,9,10,13 and is minimally elevated among women treated after age 40.3,5,9,10
Mantle RT is also associated with an increased risk of lung cancer, although the absolute excess risk is small in the first 20 years after exposure, particularly among those treated at young ages (ie, 20-year cumulative risk < 2% among those treated before age 20 years).2,5 The risks of other solid cancers have also been shown to be elevated after RT.7,8
Cardiac disease
Radiation produces dose-dependent cardiac damage through several different mechanisms. Animal models show that radiation can incite a pro-inflammatory response that facilitates the development or destabilization of coronary artery plaques.14 In addition, intimal thickening in large vessels and microvascular damage with impairment of myocardial perfusion can be seen after radiation exposure.15
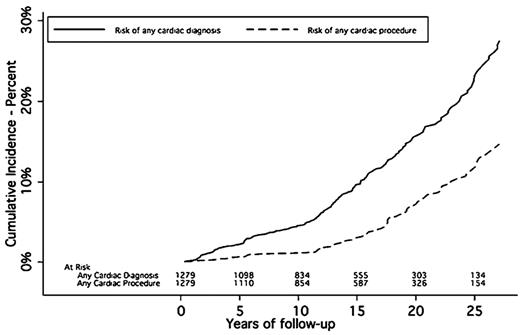
Among survivors treated with mantle RT at doses of 35-45 Gy, the SIR of significant cardiac morbidity is elevated by approximately 2- to 4-fold and the cumulative risks of significant heart disease among survivors of adult HL are approximately 5%-10% at 15 years,16 16% at 20 years,17 and 34% at 30 years18 (Figure 3). Coronary artery disease is the most common form of cardiac morbidity among HL survivors, accounting for approximately 40%-50% of adverse cardiac events. Valvular disease is less common, typically has a late onset (> 10 years after RT), and is related to higher doses (> 30 Gy) or young age at treatment. Treatment of a large cardiac volume to high dose can produce acute pericarditis, although this is uncommon. At times, this may lead to chronic or delayed reemergence of pericarditis with effusion.
Cumulative incidence of cardiac diagnoses and cardiac procedures among 1279 HL patients treated from 1969-1989. Median mediastinal dose was 40 Gy; 89.4% received > 36 Gy. (From Galper et al.17 Reprinted with permission. Copyright 2011, American Society of Hematology. All rights reserved.)
Cumulative incidence of cardiac diagnoses and cardiac procedures among 1279 HL patients treated from 1969-1989. Median mediastinal dose was 40 Gy; 89.4% received > 36 Gy. (From Galper et al.17 Reprinted with permission. Copyright 2011, American Society of Hematology. All rights reserved.)
In most studies, the combined use of doxorubicin with mantle RT has been associated with a greater risk of late cardiac toxicity than either treatment given alone.16,18 Myrehaug et al reported that for males treated at age 40, the estimated 15-year rate of cardiac-related hospitalization was 9.8% after mediastinal RT alone (SIR = 1.92) and 16.5% after combined doxorubicin with mediastinal RT (SIR = 2.80).16 Aleman et al, found that the addition of anthracyclines to mediastinal RT significantly increased the risk of congestive heart failure (relative risk = 2.81 vs mediastinal RT alone) and valvular disease (relative risk = 2.10), but did not increase the risk of myocardial infarction.18
Studies evaluating traditional cardiac risk factors (eg, diabetes, elevated cholesterol, smoking, and hypertension) have generally found them to increase the risk of heart disease among HL survivors, although there is no agreement on whether they produce supra-additive enhancement of treatment toxicity or vice versa. Aleman et al reported that conventional risk factors increased the risk of cardiac events among HL survivors, but they did not find an interaction between cardiac risk factors and HL treatment. In a study of 352 patients undergoing mediastinal RT, Glanzmann et al found that the risk of cardiac events was significantly increased only among those with cardiac risk factors.19 Similarly, 2 other studies found that all patients who developed coronary artery disease after mediastinal RT had at least one conventional risk factor.20,21 One study found that preexisting heart disease was the strongest predictor of posttreatment cardiac hospitalization (hazard ratio = 3.98) and significantly modified the effect of mediastinal RT on subsequent cardiac risk. Treatment with mediastinal RT plus doxorubicin-based chemotherapy was associated with a 10-year incidence of cardiac hospitalization that was > 20% higher than treatment with chemotherapy alone in patients with pre-HL heart disease.22
Late effects: contemporary treatment
Second cancers
Retrospective studies describing the late effects of 40-45 Gy of mantle/extended-field RT with or without alkylator chemotherapy have helped to identify groups of survivors who may benefit from early cancer screening or enhanced cardiac surveillance, and have been the motivation for developing clinical trials that are less reliant on the use of RT (and alkylating agents). However, they have limited value in predicting the risk of late effects of modern therapy. Since the implementation of 36-45 Gy of extended-field and subtotal nodal RT, there have been several important improvements in RT delivery that are expected to substantially reduce the risk of late effects. Contemporary involved-field RT (IFRT) treats only initially involved lymph node regions (± immediately adjacent regions in some cases), and prescribed RT doses are typically 30 Gy for adults and 21 Gy for children (Figure 2). The results of the German Hodgkin Study Group (GHSG) HD10 trial allow favorable-risk adult patients to receive 20 Gy of IFRT. In the majority of patients with mediastinal disease, IFRT excludes the axillae, thereby reducing breast and lung dose. It is now rarely necessary to irradiate the spleen, which was a source of a significant dose of radiation to the left breast, lung, and heart.
These changes have been shown to significantly reduce the normal tissue exposure associated with RT,23 and early clinical studies of more limited-field RT suggest that the volume reduction translates into a clinically significant reduction in SC risk.10,24 For example, a dosimetric analysis of patients receiving mediastinal RT found that the transition from mantle-field to mediastinal IFRT resulted in an approximately 65% reduction in the mean breast tissue dose among females, largely due to the exclusion of the axillae.23 Mean cardiac doses are also reduced by approximately 30%, although the dose to the proximal coronary arteries was not substantially reduced for most patients.23
The available evidence indicates that the decrease in normal tissue dose arising from the transition to IFRT should translate into reduced risks of SCs. Studies examining the dose-risk relationship for solid tumors suggest a decrease in risk with decreasing dose below approximately 40 Gy.25–27 For example, a large case-control study of young women treated for HL26 found the risk of breast cancer to be 8-fold increased (95% confidence interval, 2.6-26.4) for the highest dose category (median dose of 42 Gy) compared with the lowest one (< 4 Gy; P < .001).26 Similarly, a case-control study of breast cancer in a cohort of 6647 female survivors of childhood cancer also reported a linear increase in breast cancer risk with increasing doses up to 40 Gy.25 These results suggest that reductions in breast tissue dose below 40 Gy should produce a reduction in subsequent breast cancer risk. Similarly, the risk of lung cancer also rises with increasing radiation doses up to 40 Gy and with an increasing volume of lung irradiated28 ; similar findings have been reported for bone sarcomas and gastrointestinal cancers.29,30 One exception to these general observations is radiation-induced thyroid cancer: dose-risk studies have suggested a leveling or decrease in thyroid cancer risk with doses above 10-30 Gy.31 These findings suggest that the decrease in the volume of irradiated tissue with IFRT, and the resulting decrease in normal tissue dose, should translate into a roughly proportional reduction in the risk of most forms of SCs.
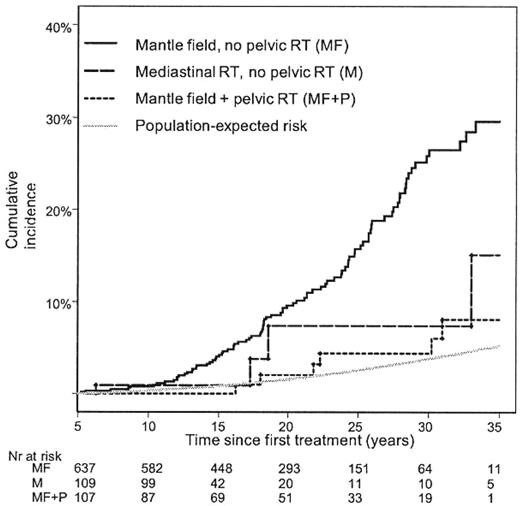
Clinical evidence is emerging that supports the concept that volume-related reduction in breast exposure will translate into a reduced risk of subsequent breast cancer. A recent study of 1122 female 5-year survivors of HL examined the effect of radiation field size on the risk of breast cancer after treatment for HL.10 Mantle RT was associated with a 2.7-fold increased risk of breast cancer compared with mediastinal IFRT of comparable prescription dose (36-44 Gy).10 The cumulative incidence of breast cancer was substantially lower after IFRT (Figure 4), although the number of patients at risk was small. This finding suggests that contemporary mediastinal IFRT will translate into lower breast cancer risks.10,24
Cumulative incidence of invasive breast cancer according to radiation fields and population-expected risk. (From De Bruin M et al.10 Reprinted with permission. Copyright 2009, American Society of Clinical Oncology. All rights reserved.)
Cumulative incidence of invasive breast cancer according to radiation fields and population-expected risk. (From De Bruin M et al.10 Reprinted with permission. Copyright 2009, American Society of Clinical Oncology. All rights reserved.)
The results of GHSG HD10 support the use of 20 Gy of IFRT after 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) in favorable-risk patients, demonstrating that further reductions in normal tissue exposure are currently possible by reducing the prescribed dose. Female patients treated with 20 Gy of mediastinal IFRT are typically receiving mean breast doses 80% lower than that received by patients treated with mantle RT in historic series, and cardiac doses are approximately 60% lower. It is important to recognize the distinction between reducing prescribed dose to large volumes and limiting RT volumes. A recent study of children treated from 1970-1990 with “low-dose” RT (15-35 Gy) reported the 20-year risk of developing a SC was 17%, indicating that the reduction in prescribed dose alone had not adequately succeeded in reducing SC risk.32 However, the RT fields studied reflected the practice at the time, and encompassed more normal tissue than contemporary IFRT treatment. Among the 15 patients who developed SCs, 14 were treated with mantle fields, including 6 with additional abdominal RT and 2 receiving additional lung RT. These findings, together with the results described above, suggest that in addition to reducing prescribed dose, restricting RT volume is an important component of reducing normal tissue exposure.
Cardiac disease
For patients with mediastinal HL, IFRT reduces the overall cardiac dose compared with mantle RT, but not necessarily the dose to the proximal coronary arteries. This relates to the initial descriptions of IFRT, which included hilar and subcarinal lymph nodes for all cases with mediastinal disease, even if these sites were not involved. Treatment of these regions would typically encompass the superior 1/3 of the heart. Therefore, whereas IFRT may reduce the morbidity caused by damage to the valves and the microvasculature within the myocardium, it is not clear that the risk of coronary artery disease will be substantially reduced among patients receiving mediastinal treatment. Further, because all contemporary patients are exposed to anthracyclines, it is plausible that cardiac morbidity may surpass SCs as the dominant late effect among survivors of contemporary HL therapy.
Involved-node RT
IFRT treatment fields are typically designed to encompass the lymph node regions (as described by Kaplan) that initially contained enlarged nodes at the time of diagnosis. Involved-node RT (INRT) extends the rationale that the benefit of RT arises from the eradication of disease that persists after chemotherapy in lymph nodes that were initially enlarged, and that it is not necessary to treat the entire lymph node region. INRT fields encompass the postchemotherapy volumes of the initially involved nodes, not the entire nodal regions.
For patients with anterior mediastinal disease, this often allows further reduction in normal tissue dose compared with IFRT due to the exclusion of uninvolved hila and subcarinal nodes (Figure 2). A small study comparing normal tissue doses delivered with IFRT and INRT found that the latter produced an average reduction in mean heart dose of 50% and in breast dose of 42%, in addition to reductions in dose to the lung, thyroid, and total body dose.33 A similar study in a series of pediatric HL patients found that mean breast and lung dose were reduced by 30% and 50%, respectively.34 It should be noted that the normal tissue sparing of INRT will be highly individualized depending on the distribution of disease: patients with disease that does not include the axillae, hila, or subcarinal nodes will benefit more from the omission of these sites from the RT fields.
It is a matter of controversy whether INRT volumes should be the “standard of care.” In one study, Campbell et al generously defined INRT as the involved nodes plus a 1.5- to 5-cm margin (ie, “INRT ≤ 5 cm”) and found no evidence of an increased risk of relapse compared with IFRT.35 The ongoing European Organisation for Research and Treatment of Cancer (EORTC) H10 study is using INRT encompassing involved nodes with a 1-cm margin. Basic geometric principles suggest that the 0.5- to 4-cm differences in the peripheral margin around an irradiated target in the EORTC definition compared with that reported by Campbell et al could translate into substantially different volumes of normal tissue irradiated. It seems improbable that randomized data comparing INRT with IFRT will be available in the foreseeable future, and expert clinicians vary in the utilization of INRT as a “standard” treatment. It is clear that excellent pretreatment imaging, patient immobilization, and image-guided delivery of RT are necessary to delineate appropriate INRT volumes. A relatively new development, it will be many years before an associated reduction in late toxicity will be clinically demonstrable.
Implications for survivors
To ameliorate the morbidity associated with the late effects described above, early initiation of cancer screening is recommended for selected survivors.36 Whereas the details of screening recommendations vary, women who have received mantle (or whole-lung) RT should have breast cancer screening initiated approximately 8-10 years after RT or by age 25-30, whichever comes later. Breast MRI should be considered for younger patients (age 30-40) due to the mammographic density of breast tissue in young women. The absolute risk of colorectal cancer screening among HL survivors is increased, although the onset of this risk is delayed compared with breast cancer.8 Some expert groups recommend that patients who received abdominal RT doses ≥ 25 Gy (eg, for para-aortic RT) should consider colorectal cancer screening 10 years after treatment or by age 35, whichever comes later. Cardiac risk factors (eg, blood pressure and serum lipids) should be monitored and, if elevated, treated appropriately among survivors. Some investigators have reported that stress echocardiography and radionuclide perfusion imaging performed 5-10 years after doses of > 36 Gy of mediastinal RT will detect significant wall-motion abnormalities or perfusion defects in approximately 10%-20% of survivors.37
Late effects: emerging developments
Intensity modulated RT, volumetric modulated Arc therapy, proton therapy
Intensity modulated radiation therapy (IMRT) refers to the delivery of RT in which treatment beams are divided into “beamlets” of varying intensity to increase the dose deposited in the tumor volume while reducing dose to adjacent normal tissues. Compared with “conventional” RT, IMRT facilitates the more conformal shaping of the high dose volume around irregularly shaped targets. Modulation of the beam intensity is often (but not necessarily) done in conjunction with the use of multiple beam angles. Volumetric modulated arc therapy (VMAT) also delivers radiation with varying beam intensity; the major distinction from IMRT being that the linear accelerator rotates through one or more arcs with the radiation continuously on, thereby reducing the treatment time. The major limitation of these techniques is that the use of multiple beam angles causes larger volumes of normal tissue to be exposed to low doses (ie, 2-10 Gy) than when anterior and posterior beams alone are used. This is of greatest concern among young females with mediastinal disease, in whom increasing the breast dose is not desirable. IMRT and VMAT are likely to be of greatest clinical advantage to male patients with mediastinal disease, in whom they facilitate significant reductions in cardiac dose.38,39 Early RT planning studies indicate that the smaller target volumes used for INRT could significantly enhance the relative merits of IMRT and VMAT.33,40
In contrast to conventional X-ray (photon) RT, proton beams have relatively little side scatter, and proton beams of a given energy have a specific range of penetration in tissue with little dose delivered beyond that distance. Consequently, in selected circumstances, proton therapy can significantly reduce the volume of normal tissue receiving low and intermediate doses. For patients with HL, one study found that, compared with photon RT, proton therapy reduced the breast dose for women with anterior mediastinal disease.41 The major limitation of proton therapy is the complexity and uncertainty of the treatment planning and the achievement of homogeneous dose, particularly for large, irregularly shaped targets.
Response-adapted selection of patients for RT
Response-adapted therapy is an emerging concept in which treatment is limited or intensified according to the early response to the initial 2-3 cycles of chemotherapy. The recently completed Children's Oncology Group (COG) AHOD 0031 trial randomized intermediate-risk patients who had a rapid early response (> 60% reduction in the product of perpendicular diameters of lymphoma mass on computed tomography) after 2 cycles of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphmide) chemotherapy and a subsequent complete response after 4 cycles of either 21 Gy of IFRT or no further therapy. No significant difference in 3-year disease-free survival was found with the addition of RT in rapid early responders, suggesting that this approach may identify the patients who can be treated safely without RT. Caution is warranted, however, as the COG AHOD0431 low-risk study also omitted RT based on radiologic complete response to 3 cycles of AV-PC (doxorubicin, vincristine, prednisone, cyclophosphamide) chemotherapy, and was closed to accrual due to an unexpectedly high relapse rate. Similarly, interim analysis of the EORTC H10 study of stage I/II patients found that it was “unlikely” that chemotherapy alone would produce disease-free survival as good as combined modality treatment in patients with negative PET scans after 2 cycles of ABVD, and it was recommended that all such patients receive combined modality therapy. These results highlight the need to evaluate reduced therapy in the context of a clinical trial rather than on an ad hoc basis. Nevertheless, response-adapted therapy is emerging as a potential means of avoiding RT for patients who may not benefit from it.
Identification of patients at risk for treatment-related toxicity
As the biologic correlates of treatment-related toxicity are better understood, it will become increasingly possible to modify treatment or use chemoprophylaxis to prevent or reduce late effects. Basic contemporary examples of this include conventional cardiac risk factors and pre-existing heart disease, which appear to be important contributors to the adverse cardiac outcomes of survivors. Recent studies have found that genetic polymorphisms involved in the regulation of intracardiac anthracycline metabolism and handling of reactive oxygen species are significantly associated with the development of anthracycline-induced congestive heart failure, suggesting a means to reduce anthracycline-related toxicity by limiting the exposures of individuals at greatest risk.42,43 RT-related tissue injury occurs through multiple mechanisms, including free radical production leading to DNA damage. Currently, there are no clear genetic correlates of the most common forms of RT-related late toxicity. With 30 years of follow-up, most patients exposed to large RT fields and doses do not develop a SC; the identification of biologic contributors to SC risk (eg, immune surveillance and DNA repair deficiencies) may help to distinguish the patients who develop SCs after RT from those who do not. In addition, observing the SC risk among patients treated with ABVD alone may help to clarify the extent to which host factors contribute to this risk.
Chemoprophylaxis
Chemoprophylaxis to prevent acute toxicity has been in use for decades. More recently, there has been an interest in the use of drugs that mediate the tissue damage caused by chemotherapy and RT as a means of reducing late effects. Animal models have been used to test potential chemoprevention strategies to reduce RT-related cardiotoxicity. Agents evaluated include ibuprofen, captopril, amifostine, and a combination of pentoxifylline and alpha-tocopherol.15 Some investigators have speculated that the pro-inflammatory response induced in cardiac tissue by RT could be attenuated with nitrogen-donating acetylsalicylic acid or statin drugs. The long latency between exposure and outcome remains the major challenge to developing these methods of reducing late toxicity
Late effects of chemotherapy
As noted above, few studies have evaluated the long-term cardiac outcomes of patients treated with ABVD. Anthracyclines are known to produce asymptomatic echocardiographic abnormalities among some pediatric patients, the clinical significance of which is not well understood. Swerdlow et al44 found that treatment with doxorubicin without supradiaphragmatic RT was associated with a significantly increased risk of fatal myocardial infarction (standardized mortality ratio = 3.2; P < .001) and ABVD was associated with a 7.7-fold increased risk (P = .01), although the median follow-up in this group was only 2.7 years. Vincristine was also associated with a significant 2-fold increase in the risk of myocardial infarction. In contrast, 2 recent studies16,18 did not find increased risks of late cardiac toxicity among young adults initially treated with chemotherapy alone, although the number of patients treated with ABVD in these series was relatively small.
Conclusion
Cohort and case-control studies of HL survivors have helped reveal opportunities to improve treatment, identify patients who should be screened for preclinical late effects, and understand the relationship between RT dose and SC risk. Substantial changes in the chemotherapy and RT used to treat HL patients over the last 10-20 years will result in very different side-effect profiles than that experienced by patients treated in most published studies of late effects. As the risk of late effects are reduced, increasingly sophisticated judgments (and larger trials) will be required to find patients who can have further treatment reductions without compromising cure.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
David Hodgson, MD, MPH, FRCPC, Department of Radiation Oncology, Princess Margaret Hospital, 610 University Avenue, Toronto, ON, M5G 2M9; Phone: (416) 946-2919; Fax: (416) 946-4586; e-mail: david.hodgson@rmp.uhn.on.ca.