Abstract
Allogeneic HSCT is performed for a small number of inborn errors of metabolism (IEM). Over the last years, transplantation outcomes have improved in this group of patients as the factors that predicted for poor transplantation outcomes were understood and addressed. The role of transplantation and its potential benefit for an individual patient with a certain IEM is therefore now much better defined. In parallel with improvements in transplantation techniques, other therapies such as pharmacological enzyme replacement therapy (ERT), substrate inhibition, and gene therapy have been developed and are increasingly available to clinicians and their patients. This review covers the following areas: (1) the scientific principles that underpin transplantation in IEM; (2) the variables of the transplantation process itself that predict for successful outcome in terms of engrafted survival after HSCT; (3) the reasons that some apparently phenotypically similar disorders might respond very differently to transplantation therapy; (4) the factors that currently influence the response of a particular patient with a particular disease to allogeneic transplantation, and how these factors might be manipulated in the future to further improve transplantation outcomes in different metabolic illnesses; and (5) how other therapeutic modalities, including ERT, gene therapy, and substrate reduction therapy, might complement and compete with HSCT in the coming years.
Scientific principles of efficacy of HSCT in IEM
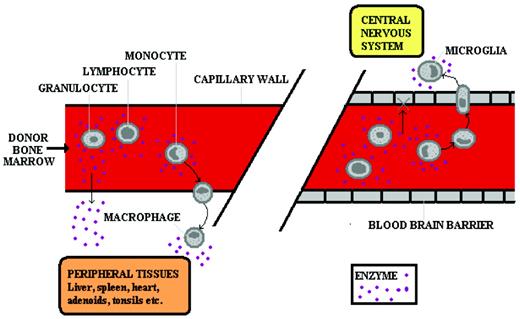
The scientific principle that underpins successful HSCT in most inborn errors of metabolism (IEM) is that of cross-correction. Engrafted donor leukocytes in the host tissue make and secrete the deficient enzyme, which is then taken up by residual enzyme-deficient host cells (Figure 1). That host cells can take up secreted deficient enzyme was first shown in cocultures of Hurler and Hunter fibroblasts, in which secreted enzymes from the fibroblasts of one disease could correct substrate accumulation in the fibroblasts of the other disease.1
Cross-correction in IEM. In the peripheral circulation (left panel), enzyme is delivered to the tissues either by diffusion of plasma enzyme (derived from pharmacologically delivered enzyme or from secretion by donor leukocytes) or by leukocytes leaving the circulation and entering the tissues. In the CNS (right panel), enzyme is delivered by the latter means only because the blood-brain barrier does not permit diffusion of the plasma-derived enzyme. Therefore, ERT does not correct CNS disease, but HSCT does.
Cross-correction in IEM. In the peripheral circulation (left panel), enzyme is delivered to the tissues either by diffusion of plasma enzyme (derived from pharmacologically delivered enzyme or from secretion by donor leukocytes) or by leukocytes leaving the circulation and entering the tissues. In the CNS (right panel), enzyme is delivered by the latter means only because the blood-brain barrier does not permit diffusion of the plasma-derived enzyme. Therefore, ERT does not correct CNS disease, but HSCT does.
These scientific observations led to the first transplantations for Hurler syndrome by Jack Hobbs in the United Kingdom 30 years ago.2 Most subsequent transplantations in IEM have been for Hurler syndrome (MPSIH), and it remains the most common IEM for which HSCT is indicated and performed and about which most is understood in terms of factors determining both graft and disease outcome.3
Other IEMs treated by HSCT include other mucopolysaccharide disorders such as Maroteaux Lamy syndrome (MPSVI), other lysosomal storage disorders (LSDs), and the peroxisomal disorder X-linked adrenoleukodystrophy (X-ALD). The mechanism of transplantation efficacy in X-ALD is not by cross-correction of enzyme-deficient host cells by secreted enzyme, so this condition will be discussed separately at the end of this review.
Hurler syndrome
Hurler syndrome arises from a genetic deficiency of α-L-iduronidase, a lysosomal enzyme that degrades the complex macromolecular glycosaminoglycans of heparan and dermatan sulfate.4 The disease-causing mutations within the iduronidase gene are described in many patient cohorts and there is a clear genotype-phenotype correlation, so those mutations that allow some residual enzyme activity give rise to phenotypically milder disease than those that have no enzyme activity. There is therefore a spectrum of MPSI disease, and Hurler syndrome is the most severe. The milder disease is known as Scheie syndrome (MPSIS) or the more severe Hurler-Scheie syndrome (MPSIHS). In general, the natural history of the condition in a particular child is predictable from the age at presentation, with more severe disease presenting in early life, and from the genotype. The level of detectable enzyme is not clinically useful. In some cases for which previously unreported mutations are involved, it may be more difficult to predict the clinical natural history and therapeutic decisions are therefore more challenging.
Hurler syndrome is a progressive, multisystem disorder that leads to premature death in childhood.5,6 There are somatic manifestations such as hepatic and splenic enlargement and soft tissue deposition, including within the coronary arteries, heart valves, and airway. Cardiac manifestation is the cause of death in childhood if the disease is untreated. There is CNS manifestation, with initially normal development before the loss of acquisition of new skills and finally loss of acquired skills (dementia). There is also an associated bony disease known as dysostosis multiplex, which includes a thoracolumbar kyphotic gibbus and hip and knee joint dysplasias that may limit mobility. In general, somatic manifestations of Hurler syndrome are best corrected by HSCT. The bony aspects of the disease are the least well corrected, although transplantation does have some effect in promoting growth and will prevent cervical instability compared with children not receiving transplantation.7,8
Hurler syndrome and HSCT
This is the paradigm disorder of HSCT in IEM. Registry studies in the 1990s described “alive and engrafted” results of approximately 45% for unrelated donor and approximately 75% for sibling donor transplantation.9,10 More recent studies have shown that “alive and engrafted” results in excess of 80% might be anticipated even after unrelated donor HSCT,11–13 and the factors that predict for successful engraftment have been described recently in a multicenter retrospective analysis.14,15 These factors are disease specific and are in addition to those factors such as improved tissue typing, larger panels of unrelated donors, better supportive care, which have contributed to the improvements in BM transplantation outcome that we see across all diseases and all age groups. Factors predicting for successful engraftment are as follows:
Full-intensity preparative regimen
Reduced-intensity procedures are associated with an increased risk of graft failure. The Inborn Error Working Party of the European Group for Blood and Marrow Transplantation (EBMT) has recently modified its recommended conditioning protocol for Hurler syndrome to fludarabine and busulfan in place of the previously used cyclophosphamide and busulfan. Serotherapy is also given depending on the donor cell source and the relationship between donor and recipient.
Busulfan with pK-guided dosing
Busulfan with pK-guided dosing is especially important in young children and infants receiving conditioning therapy and further emphasizes the importance of full-intensity conditioning therapy.
Absence of in vitro T-cell depletion of the donor marrow
Cord blood is increasingly used as a donor cell source. It is apparent that cord blood–engrafted recipients are more likely to be fully donor chimeric than their marrow-engrafted control subjects and that the outcome is particularly good in well-matched cords. The time from diagnosis to transplantation is likely to be reduced in unrelated cord transplantation recipients compared with unrelated marrow recipients, and this is likely to be important in a progressive disorder. For these reasons, cord blood might be considered an optimal donor cell source for this condition.
Long-term follow-up
HSCT is not a cure for Hurler syndrome. Hurler syndrome after transplantation is essentially a new medical condition, and we are learning more about it all the time as more patients receive transplantations. Such patients should be seen in a clinic that includes a BM transplantation physician, an endocrinologist, a metabolic disease specialist, an orthopedic specialist, and a spinal surgeon. Patients should receive a detailed annual assessment that includes additional neuropsychology testing, ophthalmic review, cardiac review, ear, nose, and throat review, and neurological and skeletal imaging. Patient support and advocacy groups should attend these assessments because there are many shared experiences that will benefit the individual patient.
Factors determining disease outcome after HSCT in Hurler syndrome and other LSDs
Transplantation outcomes have traditionally been expressed in terms of survival and engrafted survival, but transplantation is performed to ameliorate and improve an underlying disease. However, because engrafted survival rates have improved for the reasons discussed above, there is now a greater emphasis on disease outcome in a patient who has survived the procedure and remains donor cell engrafted.
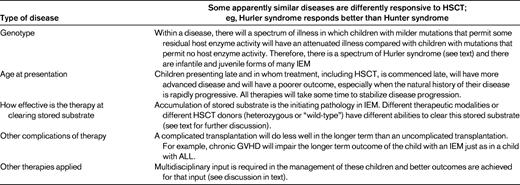
One of the difficulties of transplantation in this field of medicine is the variable outcome between apparently similar illnesses and, even within a responding illness, the variable response between different organs within any individual who has received a transplantation. We have learned the most about what influences outcome from the results of HSCT in Hurler syndrome, but over the last decades we have learned about outcomes of HSCT with many other IEMs as well.
There are 6 factors that influence outcome after HSCT in Hurler syndrome and similar LSDs for which treatment relies on cross-correction of host enzyme deficiency by secreted donor enzymes.16 Understanding these factors will allow their future manipulation where possible so that disease outcome after HSCT might be improved. These 6 factors are described below and summarized in Table 1.
(1) What is the disease?
Some disorders are apparently more sensitive to transplantation than others. Hurler syndrome responds to transplantation, but Hunter syndrome (MPSII), a clinically similar disorder that responds similarly in vitro to cross-correcting exogenous enzyme treatment, responds poorly to HSCT.
(2) What Is the genotype of the underlying illness?
In general, there is a genotype-to-phenotype relationship in IEM illnesses. Within any given disorder, those mutations (eg, premature stop and frameshift mutations) that are associated with no residual enzyme are those that are associated with the most severe phenotype. Those that have residual enzyme (eg, nucleotide substitution mutations) are associated with a milder phenotype.
(3) How old is the patient and how advanced is the disease at presentation?
Children who receive transplantations at a young age will do better than those undergoing the procedure at older ages. In older children, organ damage is established and will not be reversed with HSCT. Such observations support interventions that promote early diagnosis and early transplantation, including newborn screening programs.17
(4) How effective is the therapy at reversing substrate accumulation?
The pathophysiology of these illnesses is complex and poorly understood and is beyond the scope of this review. However, the first step is inherited enzyme deficiency and subsequent substrate accumulation. In Hurler syndrome, heparan and dermatan sulfate accumulation follows from αL-iduronidase deficiency.18 Different therapies are more effective at clearing this accumulated substrate. It is a reasonable assumption that treatments that better clear accumulated substrate will better correct the underlying disorder.
We have shown in Hurler syndrome that after transplantation from an unrelated donor, accumulated substrate is cleared better than when a carrier donor is used (such a donor has only half the genetic capability to deliver cross-corrected enzyme to residual enzyme–deficient host cells), and that transplantation from either donor is significantly better than when enzyme is delivered as pharmacological enzyme-replacement therapy (ERT).19 These data are summarized in Figure 2.
Treatment outcomes for MPS I patients. Comparison of substrate reduction, expressed as the ratio of accumulated dermatan sulfate (DS) to nonaccumulated chondroitin sulfate (CS),18 in patients who have received transplantation from a heterozygous donor, transplantation from an unrelated donor, and pharmacological ERT. The metabolic correction is better for those receiving transplantation from an unrelated donor than for those receiving transplantation from a heterozygous family donor, reflecting the better enzyme delivery by the donor with 2 copies of the enzyme gene. The metabolic correction is lowest for the ERT-treated patients and, presumably, the enzyme delivery is least good for these patients.
Treatment outcomes for MPS I patients. Comparison of substrate reduction, expressed as the ratio of accumulated dermatan sulfate (DS) to nonaccumulated chondroitin sulfate (CS),18 in patients who have received transplantation from a heterozygous donor, transplantation from an unrelated donor, and pharmacological ERT. The metabolic correction is better for those receiving transplantation from an unrelated donor than for those receiving transplantation from a heterozygous family donor, reflecting the better enzyme delivery by the donor with 2 copies of the enzyme gene. The metabolic correction is lowest for the ERT-treated patients and, presumably, the enzyme delivery is least good for these patients.
(5) What are the complications of the applied therapy?
A complicated transplantation will predict for a poor outcome regardless of the underlying disorder. A child with Hurler syndrome with severe GVHD or pneumonitis and oxygen dependence will not have the same outcome as a child with an uncomplicated transplantation, regardless of enzyme levels, substrate clearance, or chimerism.
(6) What other therapies are applied by the multidisciplinary team?
Even in those disorders that respond well to HSCT, this procedure is not a cure for all aspects of the illness, it simply ameliorates the condition (Table 1). For any child to respond as well as possible to the transplantation, the input of the entire multidisciplinary team is required in that follow-up. Conductive hearing loss due to the accumulation of middle ear fluid should be corrected with grommets. Speech therapy should be prescribed. Orthopedic and spinal surgery, judiciously and expertly applied, may influence a patient's continuing ability to walk.20 This necessity of multidisciplinary expert input supports the centralization of such patients in centers of expertise in this field of medicine, as much for the follow-up as for the transplantation process itself.
The concept of delivered enzyme to correct metabolic illness with HSCT
In considering the treatment of metabolic illness in cases in which efficacy relies on the delivery of enzyme from wild-type (donor) leukocytes to residual enzyme–deficient host cells by cross-correction, we have developed the concept of “delivered enzyme” to explain this observed variable response of different diseases to HSCT and of different organs within a responding disease to HSCT. The key components of this concept are as follows.
To prevent substrate accumulation within a given disease, the enzyme delivered by either a transplantation or pharmacological ERT must reach a certain disease-specific threshold. For a responding disease such as Hurler syndrome, this threshold is reached by transplantation or ERT, but it is not reached in a nonresponding disease such as San Filippo disease (MPSIII).
Because some organs are more correctable than others, even within a responding disease, the enzyme delivery by engrafted leukocytes or pharmacological ERT must be different to different tissues of the body. Enzyme delivery to a vascular tissue such as the heart or to the reticuloendothelial system is superior to that to the bone, which is relatively resistant to improvement after transplantation. The brain is somewhere between the reticuloendothelial system and the bone for patients receiving transplantations, but in ERT the blood-brain barrier makes it particularly difficult for enzyme delivered by ERT to correct the brain.
In any organ there will be a relationship between the enzyme delivered and the degree of metabolic correction. This is shown in Figure 3 for the kidney, where the residual substrate is measured as substrate in the urine. With increasing blood enzymes after transplantation, there is a reduction in substrate. Although this is particularly easy to measure in the kidney, there will be similar curves relating the blood enzyme level to the residual substrate for all tissues; however, for each tissue, the curve will be shifted according to the tissue constant for that material. For bone, a given blood enzyme level will be relatively less efficient at correcting tissue substrate accumulation than it will in the reticuloendothelial system.
Relationship between blood enzyme level and substrate reduction expressed mathematically. With higher enzyme levels, there is better substrate reduction. At high enzyme levels, there is no further reduction is substrate so that enzyme is no longer limiting. This curve relates kidney metabolic correction, in which residual substrate is measured as the ratio of accumulated dermatan sulfate to nonaccumulated chondroitin sulfate,18 to blood enzyme, which is expressed as the average of a years' readings in a stably engrafted patient after HSCT. Similar curves could theoretically be constructed for other organs. Where correction is currently suboptimal in certain diseases and in certain organs, it might be improved by increasing the blood enzyme level. This is the basis for proposed lentiviral gene therapy of certain intractable LSDs.
Relationship between blood enzyme level and substrate reduction expressed mathematically. With higher enzyme levels, there is better substrate reduction. At high enzyme levels, there is no further reduction is substrate so that enzyme is no longer limiting. This curve relates kidney metabolic correction, in which residual substrate is measured as the ratio of accumulated dermatan sulfate to nonaccumulated chondroitin sulfate,18 to blood enzyme, which is expressed as the average of a years' readings in a stably engrafted patient after HSCT. Similar curves could theoretically be constructed for other organs. Where correction is currently suboptimal in certain diseases and in certain organs, it might be improved by increasing the blood enzyme level. This is the basis for proposed lentiviral gene therapy of certain intractable LSDs.
Which LSDs are correctable with HSCT?
There is considerable variation in practice among physicians, centers, and across continents with regard to which patients with which illnesses should be referred for and offered HSCT. The aim of any HSCT procedure must be the preservation of an acceptable and meaningful quality of life rather than engrafted survival only. In some illnesses, engrafted survival may even prolong unacceptably poor life by preventing the somatic and immediately life-threatening manifestations of an illness while allowing continuing progression of transplantation-refractory CNS disease.21
There have been several attempts to provide a working list of metabolic disease for which HSCT should be offered. A recent communication included a transatlantic consensus table22 and the current UK consensus list is shown in Table 2. We have used this list because it is prepared specifically for commissioners of transplantation services. There are factors relating to the disease and there are factors relating to the patient with that disease that influence the decision to perform transplantation. Much evidence concerning transplantation efficacy is weak because many diseases are rare and the transplantation experience is often limited to small case series.
Current UK Paediatric Bone Marrow Transplant Group indications for HSCT in children with IEM*
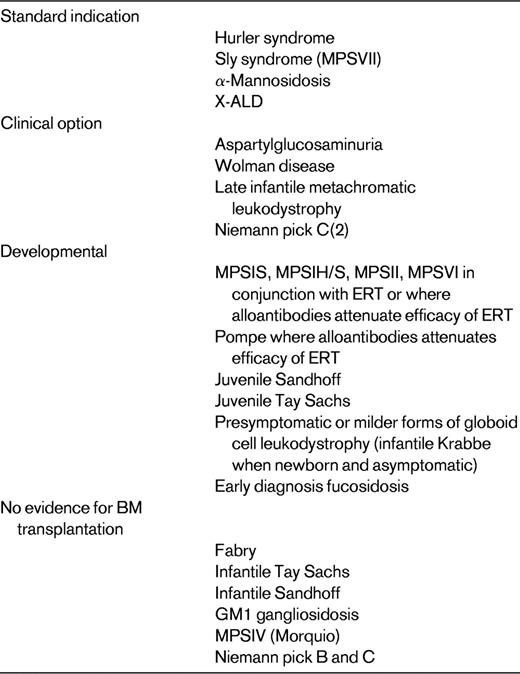
This is to guide commissioners and purchasers of HSCT services on which transplants are indicated; this is not by any means an exhaustive list and many other publications carry such a list of indications.
For those illnesses in the first column of Table 2, there is good evidence of transplantation efficacy. However, even in these illnesses a patient with advanced CNS disease (eg, one who has been diagnosed late) may not be considered a suitable candidate. For those illnesses for which transplantation is considered a “clinical option,” there is evidence from limited series to suggest that transplantation may be efficacious, but the accumulated transplantation experience is limited. It is incumbent upon the transplanting community to continue to report outcomes, including longer-term functional outcomes, in all patients receiving transplantations for a metabolic illness. Even though transplantation is considered a reasonable option in patients with these diseases, this does not mean that transplantation should be offered to all individuals with this diagnosis.
In the “developmental” stage are those illnesses for which HSCT might be expected to have some role but has not been widely applied up to this point. Pharmacological ERT has become standard of care in some of these LSDs, including milder MPSI, MPSVI, milder MPSII, and Pompe disease.23 The efficacy of such ERT might be attenuated by the development of alloantibodies against the enzyme. In Manchester, we have observed that all Hurler syndrome patients who have received ERT and undergo transplantation have very high levels of attenuating antibody at the time of the procedure. We also know that the HSCT procedure is “tolerizing” and that no patient who achieves stable, even chimeric, engraftment has long-term alloantibody detection. The incidence of such alloantibodies in patients with “milder” phenotype disease might be expected to be less, because the presence of residual host protein means that the ERT enzyme is less foreign but the actual risk of sensitization is simply not known. There are anecdotal reports of other nontransplantation methods of inducing tolerance, but the likelihood of success of such regimens remains unknown.24 In principle, in cases in which alloantibody formation limits the efficacy of ERT, then HSCT might be an option either to tolerize the patient to continued ERT or as an alternative therapy to ERT, so that the graft provides both enzyme and tolerance to that enzyme.
The differences in approach to infantile and juvenile forms of metachromatic leukodystrophy, Tay Sachs disease, and Sandhoff and globoid cell leukodystrophy simply reflect the pace of the disease. In the infantile forms of the diseases, when there is no residual protein, the pace of the disease means that diagnosis is usually only made once established disease (including disease of the CNS) is present and the window of opportunity for timely HSCT to prevent such illness is gone. In milder forms of the disease, that window of opportunity is open for longer and HSCT might be efficacious even when performed at an early age. If the diagnosis of infantile globoid cell leukodystrophy, also known as Krabbe disease, is made upon neonatal screening using the Guthrie blood spot test, then rapid HSCT using the best available umbilical cord blood donor has been shown to have some effect in ameliorating the disease phenotype. HSCT might therefore be indicated in cases for which transplantation of symptomatic patients would have been considered futile in the past.25–27
The changing therapy of IEM
Changes in HSCT, including patient selection, donor selection, conditioning, and posttransplantation care, have improved engrafted survival rates. With such an improvement in survival, transplantation is increasingly offered to patients with phenotypically milder disorders for which HSCT was not formerly offered because of the perceived risk.
The observation that outcome from HSCT will be better if the diagnosis is made earlier in the disease course has opened a debate on the role of screening for inborn metabolic errors that might respond to transplantation. The hazard to screening is the diagnosis of conditions of uncertain phenotype for which either the genotype-phenotype relationship is poorly defined or the newborn has a previously undescribed mutation and whose natural history is therefore unknown. There is a poor relationship between measured blood enzyme and disease severity. Therefore, there may be a risk of performing transplantations in individuals with milder disease who were diagnosed on the basis of a low blood enzyme level in a blood spot–screening assay because of inexact knowledge of the natural history of their disease.
In addition to improvements in HSCT therapy of metabolic disease, there are other treatments for which symptom-directed and palliative care was all that was available in the past. These therapies may complement or compete with HSCT.
ERT may be used to improve the physical condition of patients with Hurler syndrome referred for HSCT. This has been our practice in Manchester, especially in infants with very poor cardiac function.13 However, ERT should not delay the application of HSCT, and most children with Hurler syndrome who receive ERT will develop attenuating antibody in the first weeks after administration. The combination of ERT and HSCT may be relevant to other IEM, in which it may improve the clinical condition to allow the HSCT to proceed more safely.
When ERT is given to patients with metabolic disorders, these patients may develop attenuating antibodies. This is especially likely where the residual enzyme activity of the patient is low, such as in a null phenotype patient. Such patients may be “tolerized” by HSCT, as we have shown in Hurler syndrome patients who have alloantibodies to ERT administered before HSCT. In infantile Pompe disease presenting as cardiomyopathy, ERT achieves a response that is lost with subsequent, neutralizing antibody formation. Various tolerizing regimens other than HSCT have been reported in this situation, but, in principle, HSCT might also tolerize such patients to continued ERT and the relative merits and efficacy of various regimens need to be prospectively tested.
The use of multiple modality therapy for such illness might include substrate reduction therapy. In this approach, the focus is on preventing the generation of substrate rather than on accelerating the breakdown of accumulated substrate, which is the purpose of ERT or HSCT. We have recently published a study on the the efficacy of the small molecule genistein in MPSIII mice, and similar small molecule substrate inhibitors are in clinical use in other LSDs.28–30
Our observation of the relationship between delivered enzyme and metabolic outcome discussed above has been extended in other studies of transplantation therapy in these disorders. We have shown that wild-type donors achieve better correction of stored substrate than heterozygote donors. In principle, using gene-modified autologous cells, it is possible to further increase the enzyme delivery to host enzyme–deficient cells by enzyme-competent blood cells. Using such gene therapy, the number of copies of the relevant gene may be increased further beyond the wild-type or the regulation of enzyme production may be redirected using macrophage or hematopoietic-specific gene promoters. There is evidence from Biffi et al that the outcome of such gene-modified autologous cells is better than wild-type transplantation in animal models of metachromatic leukodystrophy and Hurler syndrome, and early-phase clinical trials are in process to test such results in human patients.31–33
Transplantation in X-ALD
X-ALD is a peroxisomal disorder and is the second most common indication for HSCT in an IEM after Hurler syndrome. There is very clear evidence that HSCT may improve quality and length of life in X-ALD, but, as with all HSCT in IEM, case selection and timing of HSCT is of paramount importance in this condition.34 The gene and protein is involved in the β-oxidation of long-chain very long–chain fatty acids and at least 4 distinct clinical presentations are recognized35 : (1) asymptomatic individual, in which diagnosis is made either by mutation analysis within a known family kindred or the finding of elevated long-chain very long–chain fatty acids in an asymptomatic individual; (2) adrenal insufficiency, a presentation that affects 70% of genetically affected males; (3) adrenomyeloneuropathy, a noninflammatory axonopathy presenting as progressive spastic paraparesis in young adults, including carrier females; and (4) cerebral X-ALD, a rapidly progressive, intensively inflammatory myelinopathy that most commonly begins in the corpus callosum and typically progresses to the parietooccipital region.
Transplantation is only indicated in cerebral disease, not in the other 3 presentations.36 It is not clear why transplantation is effective in X-ALD, but it is clearly not by enzyme delivery by cross-correction to enzyme-deficient host cells as in the other IEM discussed above. HSCT is usually reserved for boys who have early but definite evidence of cerebral disease as determined by magnetic resonance imaging (MRI). There is a 34-point Loes score for MRI changes in this illness that is widely used for patient evaluation.37 Patients with a score of 0 are not offered transplantation but continue to be followed with annual MRI, while boys that present with neurological disease and advanced disease on MRI are generally considered to be poor candidates for HSCT. HSCT might accelerate disease progression in such a patient, or it may stabilize a patient with a disastrously poor neurological outcome. There is no evidence that HSCT influences the other neurological manifestations of X-ALD, nor does it influence the adrenal insufficiency of these boys.
Summary
HSCT in metabolic disorders serves to change the natural history and to ameliorate multisystem disorders that present predominantly in childhood. It acts usually by providing a source of deficient enzyme from engrafted donor leukocytes, although other mechanisms such as immunosuppression and anti-inflammatory effects may also be important (in X-ALD, the mode of action is unclear). The application of HSCT to an individual patient requires consideration of: (1) the disease itself, because some diseases are more responsive to HSCT than others, and (2) the phenotype/genotype of that disease, because milder disease may not require HSCT because there are other therapeutic modalities; and (3) the disease manifestations within each individual patient, because HSCT will not usually reverse established damage but will stabilize the clinical situation. The best results will therefore be obtained in children diagnosed at the earliest stage of illness. Today, HSCT results in metabolic disorders are improved due to better understanding of those factors that influence transplantation outcome, the easy availability of cord blood, and the development of well-constructed multidisciplinary teams who deal with the many-faceted problems that children and families face. In the coming years, outcomes may be further improved by earlier diagnosis with screening and multimodality therapy including pharmacological ERT, substrate reduction, and improved cellular enzyme delivery systems using safer gene therapy techniques.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Robert Wynn, Director, Blood and Marrow Transplant Programme, Royal Manchester Children's Hospital, Manchester, United Kingdom; Phone: +44 161 701 8417; Fax: +44 161 701 8410; e-mail: robert.wynn@cmft.nhs.uk.