Abstract
One of the major efforts to improve the results of intensive therapy and autologous stem cell transplantation (ASCT) in multiple myeloma involves the integration of novel agents into the transplantation sequence. This can include their administration before, during, and after the transplantation procedure. Several phase 2 and 3 studies have evaluated the use of novel agents as part of induction therapy before transplantation to produce higher response rates and progression-free survival (PFS). Similarly, posttransplantation maintenance—or consolidation—with these agents consistently improves PFS. Survival benefits have been more difficult to demonstrate, although one trial using bortezomib before and after transplantation and a second using lenalidomide as maintenance have shown significantly longer survival times. This article reviews the different regimens used with ASCT, with an emphasis on randomized trials.
Introduction
High-dose chemotherapy and autologous stem cell transplantation (ASCT) has been an integral component of first-line therapy for younger multiple myeloma patients for more than 2 decades. For many years, this approach involved induction therapy with high-dose dexamethasone-based therapy—either VAD (vincristine, doxorubicin, and dexamethasone) or dexamethasone alone (dex)—followed by stem cell collection, high-dose melphalan, and ASCT. Data from randomized trials comparing ASCT with conventional chemotherapy demonstrated a significant benefit to ASCT in terms of progression-free survival (PFS) and, in some studies, overall survival (OS). Typically, a PFS of ∼ 2 years and OS of 4-5 years can be anticipated with such an approach.1
Several prognostic factors for outcome after ASCT have been examined. The International Staging System (ISS), which uses the β2-microglobulin and albumin levels, is applicable to stem cell transplantation recipients. In addition, FISH cytogenetics have identified the presence of t(4;14) and del17p (p53 deletion) as adverse prognostic factors; patients with these abnormalities have a median PFS of only 8-9 months and a median OS of < 2 years with a single stem cell transplantation using older techniques.2 Newer prognostic systems using gene-expression profiling and proliferative indices have also been developed. These prognostic tools can identify the ∼ 15% of newly diagnosed patients who succumb to their disease within 2 years, even when more modern transplantation regimens are used.3 Another prognostic factor for outcome, although not available at diagnosis, is the achievement of a high-grade remission post-ASCT. The achievement of complete remission (CR), near CR (nCR) (same as CR except for persistent immunofixation positivity), and/or very good partial remission (VGPR; ≥ 90% reduction in serum monoclonal protein) have each been associated with better PFS and OS in several analyses, although CR may be the most important of these.4,5 Using even more sensitive measures to detect minimal residual disease, such as multiparameter flow cytometry of BM, 2 groups have found that minimal residual disease negativity was the most important correlate of long-term PFS and OS, surpassing even immunofixation negativity in electrophoresis studies in one large Spanish study.6,7
More recently, highly efficacious novel agents, such as the immunomodulatory derivatives (IMiDs) thalidomide and lenalidomide and the proteasome inhibitor bortezomib, have been discovered and were initially used in relapsed and refractory patients. Many studies integrating these agents earlier in the disease course and in conjunction with ASCT have now been conducted and include their use in the following settings: (1) in pretransplantation induction therapy; (2) during the high-dose therapy itself; and (3) as posttransplantation measures such as “maintenance” and “consolidation” therapy. The next section focuses on the use of newer measures before and after the actual transplantation procedure, with an emphasis on the results of phase 3 studies.
Pre-ASCT induction therapy
Because numerous studies have noted that patients achieving CR, nCR, and/or VGPR after transplantation have longer remissions and survival times than those with lesser responses, it has been hypothesized that the achievement of such high-grade responses before ASCT would increase the number of patients in this favorable state after the procedure and would in turn confer a better PFS and OS. Numerous phase 2/3 trials of induction regimens capable of inducing high CR, nCR, and VGPR rates have been published, some of which are listed in Table 1.8–13 These trials have combined bortezomib and dexamethasone with conventional cytotoxic agents such as cyclophosphamide11,12 or pegylated liposomal doxorubicin,8 the novel agent lenalidomide,9,11 or both.10,11 They include the EVOLUTION trial, which is a phase 2 randomized trial comparing 4 different combinations, in which the combinations of bortezomib and dexamethasone with either lenalidomide or cyclophosphamide, but not both, yielded the best results with an acceptable toxicity profile (Table 1).11 The initial phase 1/2 study combining lenalidomide, bortezomib, and dexamethasone (RVD), conducted by the Dana-Farber group, produced an overall response rate of 100%, with a VGPR rate of 76% and a CR/nCR rate of 40%.9
Results of phase 1/2 trials of 3- and 4-drug combination induction regimens in multiple myeloma

RVDD indicates RVD + pegylated liposomal doxorubicin; VDC, bortezomib + dexamethasone + intravenous cyclophosphamide on days 1 and 8 of a 21 day cycle; VDC-mod, VCD + IV cyclophosphamide on day 15; and VDR, bortezomib + dexamethasone + lenalidomide.
*CR only.
One factor that complicates the interpretation of these trials is the lack of a standardized approach with respect to ASCT. Specifically, after several cycles, patients have the option of undergoing elective ASCT or remaining on all or some of the drugs in induction, a design that introduces considerable bias when assessing the long-term effect of these regimens. In addition, in the past, the conventional approach has been to use 3 or 4 cycles of induction and then proceed to stem cell collection and ASCT. These newer studies may report the response rates after 4 cycles, but the depth of response often continues to improve with subsequent cycles, so that the optimal duration of administration before ASCT is uncertain. Nevertheless, these 3- and 4-drug induction regimens produce very high overall remission rates, 80%-100%, with CR/nCR rates of up to 40%-50% and ≥ VGPR rates of 50%-65%. These rates are comparable to or higher than those observed after ASCT in the era before novel agents.
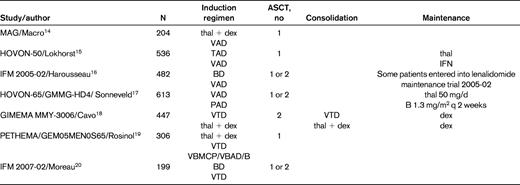
Several large phase 3 trials comparing novel combinations with older induction regimens followed by ASCT in all patients have now been reported. However, it can still be difficult to isolate the impact of novel induction regimens on the posttransplantation outcome, particularly OS, because many trials also include maintenance and/or consolidation measures post-ASCT. In addition, effective salvage therapies are usually available after disease progression in the control arm, and therefore OS may also be extended in these patients. Table 2 lists the design of several key phase 3 trials evaluating the following novel induction regimens followed by ASCT: thalidomide + dex (thal + dex) by the MAG group,14 thalidomide + Adriamycin + dex (TAD) in the HOVON-50 trial,15 bortezomib + dexamethasone (BD) in the IFM 2005-01 trial,16 bortezomib + Adriamycin + dexamethasone (PAD) in the Dutch/German HOVON MM-65/GMMG-HD4 trial,17 bortezomib + thalidomide + dexamethasone (VTD) in the Italian GIMEMA trial,18 VBMCP/VBAD/B (vincristine + carmustine + melphalan + cyclophosphamide + prednisone/vincristine + carmustine + doxorubicin + dexamethasone/bortezomib) in the 3-arm Spanish PETHEMA/GEM05MEN0S65 trial,19 and lower-dose bortezomib + thal + dex (vTD) in the IFM 2007-02 trial.20 The comparator was VAD in the first 4 trials, thal + dex in the Italian trial by Cavo et al, and BD in the most recently conducted IFM trial by Moreau et al. The results are summarized in Table 3. Although, as mentioned above, the use of posttransplantation therapy complicates the interpretation of the role of induction therapy, the post-ASCT CR/nCR and ≥ VGPR rates are unprecedented at 30%-60% and 60%-85%, respectively, and the median PFS in these trials is significantly higher than in the control arm and is in the range of 3 years. Despite these favorable findings, the only trial to demonstrate a statistically significant improvement in OS using novel agents in conjunction with ASCT is the HOVON MM-65/GMMG-HD4 study, in which bortezomib was used both before and after ASCT.17
Results of phase 3 trials of induction therapy in myeloma patients undergoing ASCT
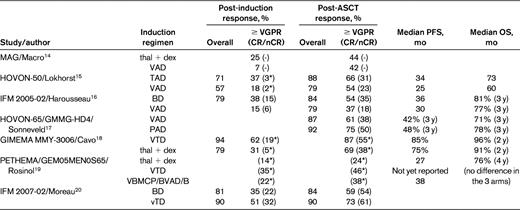
*CR rate only.
Several of these studies reported details about subset analysis. First, in the IFM 2005-01 trial, a significant advantage was observed in patients with ISS stage II-III disease who received BD versus VAD before ASCT.16 Second, improvements in PFS and OS were observed in the patients with t(4;14) who were given BD.21 The results of t(4;14) patients in this and other studies is illustrated in Table 4. Taken in aggregate, the outcomes of patients with this cytogenetic abnormality fared better with the use of a bortezomib-based induction regimen versus VAD, but the results were still less favorable than in patients without t(4;14).2,21–25 One explanation for the inferior results using alkylating agents—including high-dose melphalan—in this t(4;14) subgroup has been proposed by Scottsdale Mayo Clinic group. They found that the MMSET dysregulation that characterizes many t(4;14) patients results in aberrant responses to DNA-damaging agents such as melphalan.26 An ongoing Canadian phase 2 trial in newly diagnosed patients with t(4;14) is based on bortezomib combination therapy administered for 1 year, followed by dexamethasone maintenance without planned ASCT. Interim results indicate that the median PFS is ∼ 2 years, which compares favorably with the results of ASCT programs in t(4;14) disease.27
Results of ASCT trials in myeloma patients with t(4;14)
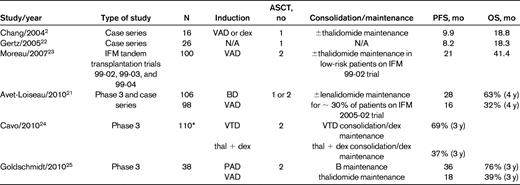
*Includes t(4;14) patients with and without del 17p.
Results in patients with del 17p have been less encouraging, and these patients continue to have suboptimal results with ASCT, even using the aggressive Total Therapy 3 program discussed below.28
Two other induction approaches merit discussion. The regimen of oral lenalidomide and dexamethasone (len + dex) is another induction regimen frequently used in the United States. It has been evaluated in a phase 3 trial comparing lenalidomide and either high-dose pulse dexamethasone (40 mg on days 1-4, 9-12, and 17-20 of a 28-day schedule) or weekly low-dose dexamethasone (40 mg) for 4 cycles in newly diagnosed myeloma patients, but subsequent therapy was not specifically mandated. The overall response rates after 4 cycles were 79% versus 68%, with ≥ VGPR rates seen in 42% versus 24%, respectively, for the high-dose and low-dose dexamethasone arms. A landmark analysis of the patients who underwent ASCT yielded a 3-year survival of 92%.29 However, the trial design predisposes to potential bias for the ASCT patients because they might represent either high-risk patients expected to do poorly with continued len + dex or, conversely, might be comprised mainly of younger and healthier individuals. Nevertheless, the available data indicate that len + dex is a well-tolerated and highly convenient first-line regimen, does not significantly compromise stem cell collection as long as patients are mobilized with chemotherapy and hematopoietic growth factors, and produces a high overall response rate after 4 cycles.
The other noteworthy approach to induction before ASCT includes the Total Therapy programs developed by the University of Arkansas myeloma group, although they have not been compared with less intensive transplantation approaches in randomized trials.30,31 In the Total Therapy 2 trial, newly diagnosed patients were treated with 2 cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide), followed by tandem ASCT, further D-PACE consolidation and maintenance with IFN for 1 year, and dexamethasone until progression. These patients were randomized to either thalidomide throughout the entire program, or to no thalidomide. Remission rates before and after ASCT were higher in the thalidomide group, and PFS was significantly longer in this arm.30 Although this advantage did not translate into a survival benefit for the thalidomide arm due to a shorter survival after myeloma progression, longer follow-up revealed that higher-risk patients (those with cytogenetic abnormalities by metaphase karyotyping) had a significant survival benefit with thalidomide.31 The next study from this group, Total Therapy 3, builds on this experience by incorporating bortezomib, thalidomide, and dexamethasone (VTD) as VTD-PACE given before and after tandem ASCT. With this approach, the response rate, PFS, and OS have improved further in newly diagnosed patients with low-risk disease as defined by gene-expression profiling.3 Nevertheless, as mentioned above, ∼ 15% of patients experience early treatment failure and death; optimal treatment of this subset remains challenging and will be the target of future cooperative trials.
Our own group at Princess Margaret Hospital has selected the 3-drug bortezomib regimen CyBorD weekly as the pre-ASCT induction regimen, consisting of weekly bortezomib 1.5 mg/m2 on days 1, 8, 15, and 22 of a 28-day schedule, along with weekly oral cyclophosphamide 300 mg/m2 and dexamethasone, the latter initially in a pulse fashion for cycles 1 and 2, and then weekly for cycles 3 and 4.12 Advantages include the lower incidence of toxicity (peripheral neuropathy and thrombocytopenia) and improved patient convenience compared with biweekly bortezomib. The cost and remission rates, including a CR/nCR rate of almost 50% after 4 cycles, compare favorably with other 3-drug bortezomib-containing regimens that contain an IMiD. However, despite the high remission rates pre-ASCT in all subgroups, it is insufficient, in the absent of post-ASCT measures, to confer a longer advantage in PFS or OS in high-risk subgroups such as t(4;14).
In summary, all of the regimens above establish the superiority of regimens containing novel agents in terms of achieving higher response rates, including CR/nCR rates, after induction therapy. Thalidomide-based regimens such as thal + dex and TAD are associated with the usual toxicities seen with this ImiD—peripheral neuropathy, constipation, fatigue, and increased risk of venous thromboembolism—and produce responses on the lower end of the spectrum. In addition, a benefit on post-ASCT status has not been established. The highest response rates are observed using 3-drug regimens, particularly those containing both bortezomib and an IMiD, and the RVD combination developed by the Dana Farber group has been selected as the test arm of several large cooperative group trials. Bortezomib-associated peripheral neuropathy, which may be painful, can occur when this drug is part of induction. The use of less intensive bortezomib dosing, either as 1.0 mg/m2 biweekly doses in the vTD regimen or 1.5 mg/m2 weekly doses in the CyBorD regimen, appears to decrease the chance of grade 3 or 4 neuropathy without compromising response rates, but only the former combination has been evaluated in a randomized fashion.12,20 In addition, antiviral prophylaxis against herpes zoster is required during induction with bortezomib to avoid the risk of reactivation during therapy.
Post-ASCT therapy
A myeloma consensus panel recently defined maintenance therapy as any treatment administered after the completion of induction therapy in patients whose disease is either responsive or nonprogressive at that time, with the goal of prolonging survival. Earlier ASCT studies used less potent agents such as corticosteroids or IFN-α with variable results and, particularly with IFN-α, toxicity concerns. The availability of more effective novel agents has generated a renewed interest in maintenance therapy in this disease, and several phase 3 trials have now been reported.
The concept of post-ASCT consolidation therapy was initially introduced into myeloma treatment as part of the Total Therapy programs developed by Dr. Barlogie at the University of Arkansas and, similar to acute leukemia therapy, referred to the administration of additional courses of intensive chemotherapy after induction, ideally after the patient had achieved a CR. However, the definition of consolidation is not standardized in myeloma, and the difference between consolidation and maintenance is not always clear. Regardless, the goal of consolidation therapy in myeloma is to improve the depth and length of posttransplantation response.1
Maintenance therapy
IMiDs
The IMiDs thalidomide and lenalidomide have been the most frequently studied maintenance drugs, given their ease of oral administration and established antimyeloma efficacy. Table 5 summarizes the 7 randomized trials of post-ASCT thalidomide maintenance. These studies differ with respect to induction regimen used, use of single or tandem transplantation before maintenance, dose and duration of thalidomide, concomitant use of corticosteroids, and comparator arm. Despite these differences, thalidomide maintenance was consistently associated with a longer PFS, although the benefit on OS was variable.15,30,32–36
Post-transplantation maintenance therapy with IMiDs
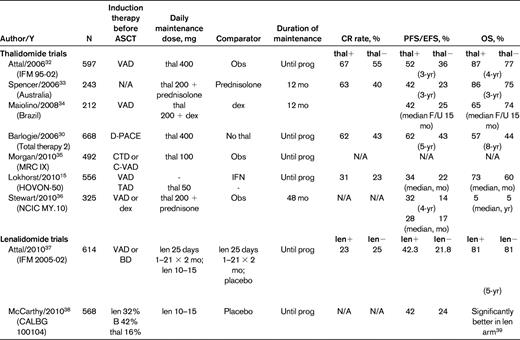
EFS indicates event-free survival; F/U, follow-up; N/A=not available; obs, observation; prog, progression.
In addition to the PFS benefit, several observations are evident from these trials. First, in some instances, thalidomide improved the depth of response and therefore functioned as a therapeutic agent, rather than simply providing a stabilizing effect on the myeloma burden.32 Second, because none of these trials mandated therapy for progressive myeloma, the possibility that heterogeneity in such treatment influenced survival outcomes has been raised. For example, a statistical exercise performed using the British MRC IX dataset, in which patients of all ages were randomized to either thalidomide or no therapy after induction therapy (which included ASCT in the younger patients), demonstrated a significantly longer survival in patients given thalidomide maintenance if they subsequently had access to novel agents at the time of myeloma progression, but not in those who were treated only with further thalidomide or older cytotoxic agents.35 Other phase 3 trials, including the Therapy 2 trial, the HOVON-50 trial, and the NCIC trial (Table 3), have attributed the absence of a survival advantage to a shorter life expectancy after relapse in patients who received thalidomide maintenance.15,30,36 The NCIC trial recorded the treatment administered at the time of disease progression and found similar access to lenalidomide and bortezomib in both arms of the study. Despite this, the OS was still not significantly improved in the thalidomide arm (not yet reached versus 5 years in the thalidomide plus prednisone vs observation arm, respectively, at a median follow-up of 4 years; hazard ratio 1.29 [95% confidence interval 0.89-1.88]; P = .188).36 This finding raises the possibility that the myeloma cell population might be intrinsically more resistant after exposure to maintenance therapy.
Several investigators have tried to identify the patient subgroups most likely to benefit from maintenance thalidomide. In a post-hoc analysis of the IFM 95002 trial, Attal et al determined that the beneficial effect of maintenance therapy was limited to patients who had achieved < VGPR and whose myeloma cells lacked the cytogenetic abnormality del13q by FISH. This trial excluded high-risk patients, because eligible patients could have no more than one adverse feature, either high β2-microglobulin or del13q.32 Conversely, the HOVON-50 trial, which compared a comprehensive program of TAD induction, ASCT, and thalidomide maintenance with another program of VAD, ASCT, and IFN maintenance, found that patients with del13 detected by conventional cytogenetics did not experience a worse outcome compared with patients lacking this abnormality when thalidomide-based therapy was used.15 More extensive FISH testing was available in patients on the MRC IX trial. The preliminarily analysis revealed that patients with del17p (p53 deletion) by FISH had a particularly poor outcome if thalidomide maintenance was used.35 Finally, an updated analysis of the Total Therapy 2 trial by the Arkansas group, which initially reported only better PFS when continuous thalidomide was added to their aggressive multiphase program, later described a significantly better survival in the thalidomide arm in patients considered high-risk due to the presence of cytogenetic abnormalities defined by conventional karyotyping.30,31 Due to the varied criteria used to subclassify patients, a definitive conclusion about the optimal use of thalidomide maintenance remains uncertain.
Despite the longer PFS with thalidomide maintenance, this approach has not gained uniform acceptance outside of clinical trials due in large part to the toxicity of this drug. Peripheral neuropathy, sedation, and constipation are common, and rashes and an increased risk of venous thromboembolism may also occur. These side effects lead to a reduction in quality-of-life parameters and premature discontinuation even when low doses are used. In practical terms, patients can usually tolerate the drug < 1 or 2 years before side effects become prohibitive.15,35,36
More recently, lenalidomide maintenance therapy has been studied, and 2 randomized trials comparing low doses of lenalidomide (10-15 mg/d) with placebo after ASCT have completed accrual: the CALGB-100104 trial in North America and the IFM 2005-02 study in France.37,38 The induction regimens were not specified in these studies, and the French trial included some patients who had had undergone tandem ASCT. In addition, the IFM trial administered the full therapeutic dose of lenalidomide (25 mg) for 2 months as consolidation to all patients shortly after ASCT and before randomization to the lower maintenance dose, whereas the CALGB study simply commenced maintenance lenalidomide at a dose of 10-15 mg on days 60-90 post-ASCT.37,38 Both of these trials reported an excellent PFS rate of 42 months in the lenalidomide arm, with a significant survival benefit now observed in the CALGB study in the most recent analysis presented at the International Myeloma Workshop in 2011.39 Toxicity was acceptable and only 6% of patients in the IFM trial and 13% in the CALBG trial discontinued lenalidomide maintenance early, before myeloma progression, in contrast to the experience with thalidomide. Neutropenia was the most common side effect noted, but febrile neutropenia was rare.37,38
An unexpected finding from the lenalidomide maintenance trials was a modest increase in the incidence of secondary cancers, including secondary myelodysplastic syndromes (MDS) and/or acute myelogenous leukemia (AML): ∼ 6.5% in both studies compared with 1.6%-2.6% in the placebo groups for all tumor types.37,38 Intense investigation regarding the risk of secondary cancers in recipients of lenalidomide in various disease settings has ensued. Interpretation of the risk is complicated by the recent recognition that new primary malignancies, particularly MDS/AML, occur with increased frequency in plasma cell disorders such as monoclonal gammopathy of undetermined significance in the absence of cytotoxic/genotoxic therapy. In addition to a suspected intrinsic risk of second cancers in this disease, antimyeloma drugs other than lenalidomide may predispose to secondary malignancies. In this regard, the IFM maintenance study identified prior exposure to the DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin) regimen as an adverse prognostic factor for the development of secondary malignancies while on lenalidomide. This regimen is, fortunately, unlikely to be used commonly in the future before ASCT, because it did not confer any improvement in posttransplantation outcome when evaluated in an IFM phase 3 trial. Moreover, other alkylating agent regimens used more often in myeloma therapy have long been known to be associated with a low but measurable risk of MDS/AML and, because myeloma patients live longer due to improved therapy, this risk is likely to become more apparent. It appears that the risk of secondary cancers in patients treated with lenalidomide is quite small and is likely counterbalanced by the strong antimyeloma efficacy of this drug when used in the maintenance setting.
The risk of secondary cancers when novel agents other than lenalidomide are used for maintenance after ASCT has not been rigorously evaluated, but appears to be low. For example, the NCIC trial reported only 1 fatal secondary cancer in the thalidomide plus prednisone maintenance arm (0.6%) versus 2 in the observation arm (1.2%).36 One other potential advantage of thalidomide over lenalidomide maintenance therapy is the anticipation that more therapeutic options will be available when relapse eventually occurs. Thalidomide-treated patients will likely subsequently benefit from both lenalidomide- and bortezomib-based regimens, because even patients refractory to thalidomide have been shown to have satisfactory remission rates and remission durations with lenalidomide. Although the data are limited, the converse may not be true, because, in our experience, patients progressing on lenalidomide who are subsequently treated with thalidomide usually experience minimal benefit. Avoiding the most effective agent in the hopes of maximizing options for relapse is not a common approach in any malignancy, however, and has not been formally tested as a treatment strategy in well-designed trials. In addition, it will be important to determine whether patients given lenalidomide maintenance will respond to increasing the dose of lenalidomide to the usual therapeutic dose and adding dexamethasone at the time of myeloma progression.
Bortezomib
The HOVON-65/GMMG-HD4 trial described above (Table 2) was the first to report results using posttransplantation bortezomib maintenance. In this study, bortezomib 1.3 mg/m2 was given every 2 weeks for 2 years after PAD induction and 1 or 2 transplantations. All outcomes were significantly better than the arm with VAD induction and low-dose thalidomide maintenance 50 mg daily. After ASCT, 57% of the patients randomized to bortezomib maintenance started the drug; 27% required dose reductions and 9% discontinued bortezomib due to toxicity. In the thalidomide arm, 67% of post-ASCT patients randomized to thalidomide maintenance started the drug, but 31% discontinued thalidomide due to toxic effects. Multivariate analysis showed that bortezomib treatment was a significant variable for OS.17
Consolidation therapy
The use of consolidation therapy after ASCT has been evaluated in 3 recent phase 3 trials. The Italian trial by Cavo et al, which has yielded some of the best outcomes of a transplantation program to date, used the VTD regimen, which was also given for induction, for 2 cycles after transplantation as a consolidation measure. The control arm was given thal + dex without bortezomib for 2 months.17 As discussed above, the IFM 2005-02 trial designated the use of single-agent lenalidomide given at the full therapeutic daily dose of 25 mg for 2 cycles as a consolidation rather a maintenance phase of treatment, followed by the lower maintenance dose of lenalidomide until progression.37 The Nordic Myeloma Study Group randomized ASCT patients at 3 months to either consolidation with single-agent bortezomib administered biweekly in a 21-day cycle for 3 cycles, followed by a weekly dosing on days 1, 8, and 15 of a 28-day cycle for 4 additional cycles or no therapy.40 The use of bortezomib resulted in a rate of ≥ VGPR of 70% compared with 50% in the arm without consolidation (P = .01); the PFS was also improved, at 27 months from the time of randomization versus 20 months in the no-treatment group (P = .02). The 2-year OS was 90% in both groups.40
These studies of post-ASCT maintenance and/or consolidation indicate that the administration of each novel agent can delay disease progression, although an OS benefit has not always been realized. Currently, the superiority of any one particular post-ASCT strategy using novel agents has not been established with absolute certainty, although the phase 3 trials of lenalidomide maintenance compared with placebo have demonstrated the longest PFS after ASCT reported to date: > 3.5 years. The ongoing Clinical Trials Network (CTN) trial in the United States will help to clarify the benefit of different posttransplantation treatments, because it randomizes patients after ASCT to proceed directly to lenalidomide maintenance versus undergoing a second ASCT followed by lenalidomide maintenance versus consolidation with RVD followed by lenalidomide maintenance. Until further information is available, the selection of the preferred approach will likely depend on issues of toxicity and patient/physician preference. In the future, information regarding efficacy in different disease subgroups and concerns regarding long-term outcomes, such as secondary malignancies and options for treatment for eventual relapse, will likely influence post-ASCT management.
Summary and conclusions
Unlike many other hematologic malignancies, several new antimyeloma drugs have been introduced and have shown efficacy in phase 2/3 trials when integrated before and after ASCT. This favorable situation, however, has produced uncertainty for hematologists regarding the best approach to select for transplant-eligible myeloma patients. In the absence of consistent and reproducible survival benefits with a given approach, the physician has considerable flexibility in choosing induction and maintenance/consolidation regimens, but should be guided by expectations of a 70%-80% high-grade remission rate (ie, ≥ VGPR) after ASCT and a median posttransplantation PFS of at least 3 years. Based on the available data, albeit limited, patients with t(4;14), and likely also those with other poor prognostic factors such as high ISS score and/or plasma cell leukemia, should receive bortezomib-based regimens at least for induction. To date, the best reported results with respect to PFS have used novel agents in induction followed by lenalidomide as maintenance after ASCT, and the ongoing CTN trial will help to determine the contribution of a second ASCT or consolidation therapy to lenalidomide maintenance. Further information is required to clarify issues regarding the management of posttransplantation relapse in patients exposed to the 2 most potent novel agents, bortezomib and lenalidomide, as part of first-line therapy, as well as to evaluate the risk of late untoward effects such as secondary malignancies as myeloma patients experience longer survival times. Innovative measures will be needed to improve the outcome of the 15% of patients with very high-risk features at diagnosis, such as del17p and/or unfavorable gene-expression profiling results.
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Otsuka, Merck, Janssen, Celgene, Bristol-Meyers Squibb, and Amgen and has received research funding from Otsuka, Merck, Johnson & Johnson, Janssen, Celgene, Bristol-Meyers Squibb, and Millennium. Off-label drug use: Lenalidomide for initial and maintenance therapy of myeloma; bortezomib for initial therapy before stem cell transplantation and as maintenance therapy in myeloma.
Correspondence
Donna E. Reece, MD, Princess Margaret Hospital, 610 University Ave, Toronto, Ontario M5G 2M9 Canada; Phone: (416) 946-2824; Fax: (416) 946-6546; e-mail: donna.reece@uhn.on.ca.