Abstract
Patient outcome in multiple myeloma (MM) has been remarkably improved due to the use of combination therapies including proteasome inhibitors and immunomodulatory drugs, which target the tumor in its BM microenvironment. Ongoing efforts to improve the treatment paradigm even further include using oncogenomics to better characterize molecular pathogenesis and to develop refined patient stratification and personalized medicine in MM; using models of MM in its BM milieu to identify novel targets and to validate next-generation therapeutics directed at these targets; developing immune-based therapies including mAbs, immunotoxins targeting MM cells and cytokines, and novel vaccine strategies; and using functional oncogenomics to inform the design of novel combination therapies. With continued rapid evolution of progress in these areas, MM will be a chronic illness with sustained complete response in a significant number of patients.
Introduction
Multiple myeloma (MM) represents a paradigm in drug development targeting tumor cells in their BM microenvironment.1–3 In particular, the observation that the proteasome inhibitor bortezomib and the immunomodulatory drugs thalidomide and lenalidomide target the MM cell in the BM microenvironment has rapidly translated from the “bench to the bedside” in collaborative trials. This has resulted in 6 new US Food and Drug Administration (FDA)–approved treatments in the past 7 years, with a doubling of patient survival from 3-4 to 7-8 years as a direct result.4 The use of these targeted inhibitors to treat relapsed refractory MM, relapsed MM, newly diagnosed MM, and most recently as consolidation and maintenance therapies, has totally transformed MM therapy and patient outcome. Oncogenomic studies, coupled with models of MM cells in the BM milieu, now allow for definition of the heterogeneity of disease, identification of novel targets in the tumor and microenvironment, and validation of inhibitors directed at these targets. Ongoing research will develop personalized therapy, immune-based therapies, next-generation targeted agents, and rationally based combination targeted therapies.
Development of personalized medicine in MM
Marked genetic heterogeneity has been demonstrated in MM, which has important implications for tumor pathogenesis, prognosis, and treatment. For example, hyperdiploid and t(11;14) mutations have defined standard-risk MM with superior outcome to conventional therapy, whereas nonhyperdiploid, t(4:14), del (17p), and del(13q14) mutations have defined high-risk MM with inferior outcome. However, novel therapies such as bortezomib can mitigate to some extent the adverse outcome conferred by these abnormalities.5 Microarray, miRNA, and gene-profiling studies such as array comparative genomic hybridization and single nucleotide polymorphism analysis of clinically annotated samples allow for characterizing the molecular pathogenesis of MM, identifying novel targets, and developing refined patient stratification and personalized medicine in MM. For example, microarray profiling has defined transcriptional changes that are correlated with the evolution from monoclonal gammopathy of undetermined significance to smoldering MM to active MM,6 and defined transcript-based prognostic MM classification systems.7–11
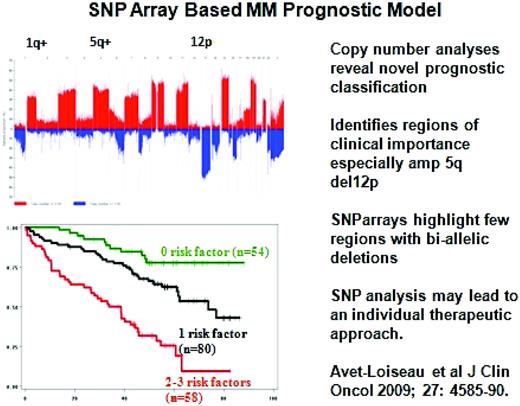
Already, genetic and molecularly distinct subgroups of MM have distinct biology and treatment options; for example, FGFR3 inhibitor therapy may be useful in t(4:14) MM and anti-CD20 mAb therapy in CD20+ MM. Microarray profiling has also defined signatures of response versus resistance to novel therapies such as bortezomib.12 Array comparative genomic hybridization studies have identified copy number alterations that both suggest potential novel MM oncogenes or suppressor genes as potential therapeutic targets and provide for DNA-based prognostic classification of MM.13 Moreover, single nucleotide polymorphism analyses of patient samples has identified copy number alterations predictive of clinical outcome, including increased 1q and 5q or decreased 12p as sites for putative MM oncogenes or tumor suppressor genes, respectively (Figure 1).14
Recurrence of copy-number abnormalities across 192 MM cases in chromosomal order. Red or blue bars denote gain or loss of chromosome material. (Modified with permission from Avet-Loiseau et al.14 )
Recurrence of copy-number abnormalities across 192 MM cases in chromosomal order. Red or blue bars denote gain or loss of chromosome material. (Modified with permission from Avet-Loiseau et al.14 )
miRNA profiling studies have distinguished normal plasma cells from MM cells, and patients with tumors resembling the former have improved outcome compared with patients whose tumors resemble the latter. Finally, the Multiple Myeloma Research Foundation MM genome sequencing studies have revealed mutated genes involved in protein homeostasis, NF-κB signaling, IRF4 and Blimp-1, and histone-methylating enzymes, all of which are consistent with MM biology.15 These studies also unexpectedly identified BRAF mutations that have also been described and targeted clinically in malignant melanoma.16 Studies have identified distinct genetic changes at the time of diagnosis versus relapse of MM, reflective either of the expansion of resistant clones already present at low frequency at diagnosis or the acquisition of new abnormalities. These data strongly support the view that personalized medicine in MM must include profiling patient tumor cells not only at diagnosis, but also at the time of relapse.
Development of next-generation agents targeting the tumor cell in its microenvironment
Oncogenomic studies, coupled with models of MM in the BM microenvironment, have defined the role of tumor cell–BM accessory cell contact and cytokines in the BM milieu in conferring growth, survival, and drug resistance in MM1–3 and have also validated targeted inhibitors. For example, up-regulation of the ubiquitin proteasome cascade at both a gene transcript and activity level in tumor cells is triggered by MM-BM binding,17 making it an even better target in this context for bortezomib, which is able to overcome cell adhesion–mediated drug resistance to conventional therapy. These model systems allow for defining the impact of agents on the tumor versus the microenvironment. For example, in MM cells, bortezomib inhibits chymotryptic proteasome activity, abrogates tumor growth and survival, induces apoptosis, up-regulates heat shock proteins, inhibits DNA damage repair, and induces ER stress. Conversely, in the BM milieu, bortezomib down-regulates adhesion molecules on tumors and BM, triggers anti-angiogenesis, and—importantly—induces apoptosis of osteoclasts while promoting osteoblast differentiation.18,19 Bortezomib was rapidly adapted into clinical practice and received accelerated FDA approval for the treatment of relapsed and refractory MM, followed by full approval in relapsed MM and as initial therapy, based upon its superiority in randomized phase 3 clinical trials.20,21 Most recently, very promising data on bortezomib as consolidation and maintenance therapy are emerging both in patients not receiving transplantation22,23 and in transplantation patients.24,25 Its ability to maintain efficacy but markedly reduce attendant neuropathy when used weekly26 or subcutaneously27 has also been shown. Gene profiling can define signatures predictive of response12 and for the development of neuropathy25 to bortezomib. In addition, functional assays can be used to show that MM cells with high proteasome load and low proteasome capacity have high proteasome stress and are therefore susceptible to proteasome inhibition. In contrast, solid tumors with high proteasome capacity and low proteasome load are relatively resistant to proteasome inhibitors.28
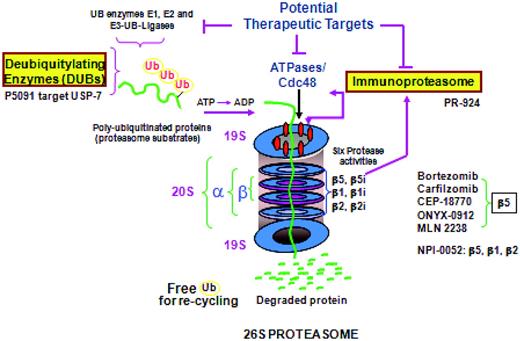
Second-generation inhibitors of the proteasome ubiquitin cascade are now in preclinical and clinical studies (Figure 2). Inhibitors of deubiquitinating enzymes upstream of the proteasome, such as the USP-7 inhibitor P5091, have anti-MM activity in preclinical studies. More potent inhibitors of chymotryptic activity (eg, carfilzomib, ONYX 0912, and MLN 9708)29–32 have been shown to overcome bortezomib resistance in preclinical and early clinical trials. For example, in phase 2 clinical trials, carfilzomib has achieved responses even in bortezomib-refractory MM without neuropathy; and phase 3 clinical trials of carfilzomib lenalidomide dexamethasone versus lenalidomide dexamethasone in relapsed MM are ongoing for new drug approval. Both Onyx 091231 and MLN 970832 are oral chymotryptic inhibitors showing promise in early phase 1/2 clinical trials. A more broad proteasome inhibitor, NPI-0052, which targets chymotryptic-, tryptic-, and caspase-like activities has been shown to overcome bortezomib resistance in preclinical studies,33 and shows early clinical promise. Finally, PR-924, an inhibitor of the LMP-7 immunoproteasome subunit, also blocks MM growth in vitro and in vivo,34 and inhibitors of the immunoproteasome should have a favorable therapeutic index due the selective expression of immunoproteasome subunits in malignant, but not normal, hematologic cells.
The immunomodulatory drugs thalidomide, lenalidomide, and pomalidamide target MM cells in the BM microenvironment. Specifically, these agents directly trigger caspase 8–mediated apoptosis; decrease binding of tumor cells to BM; inhibit constitutive and MM cell binding–induced secretion of cytokines from BM; inhibit angiogenesis; and stimulate autologous natural killer, T, and natural killer–T-cell immunity to MM cells.1–3,35 Preclinical studies have shown additive cytotoxicity of lenalidomide (ie, it triggers caspase 8–mediated apoptosis) combined with dexamethasone (ie, it induces caspase 9–mediated apoptosis), and phase 1 and 2 clinical trials have established the maximal tolerated dose and confirmed the enhanced clinical efficacy of the combination, which in turn informed the design of phase 3 clinical trials leading to its FDA/European Medicines Agency approval to treat relapsed MM.20,26,36–39 Randomized trials have established the utility of lenalidomide as initial therapy in both transplantation candidates (lenalidomide/low-dose dexamethasone)40 and elderly patients (lenalidomide melphalan prednisone)41 ; as consolidation therapy after transplantation; and as maintenance therapy either after transplantation or after lenalidomide melphalan prednisone in non-transplantation candidates, in both cases by virtue of markedly prolonged progression-free survival.3 Although secondary malignancies have been noted more commonly in those patient cohorts randomized to receive lenalidomide maintenance versus controls, the benefits of maintenance lenalidomide far outweighs this risk. Ongoing studies are examining genetic factors in the tumor and host and treatment-related factors, including lenalidomide, that are correlated with risk of secondary cancers. To date, prior DNA-damaging agent therapy with dexamethasone cyclophosphamide VP-16 and platinum has been identified as a risk factor for the development of malignancies with the use of lenalidomide after transplantation.
Therapies targeting accessory cells and cytokines that affect MM
Bortezomib and lenalidomide are agents targeting MM cells that also affect the microenvironment (ie, both stimulate new bone formation).19,44 Conversely, agents targeting the MM BM microenvironment may also have anti-MM efficacy. The MRC IX trial, for example, evaluated zoledronic versus clodronic acid in newly diagnosed transplantation and non-transplantation candidates and showed that the former not only decreased skeletal events, but also prolonged overall survival.45 Second, MM cells secrete DKK-1, which in turn down-regulates osteoblast function via targeting Wnt signaling. In preclinical murine xenograft models of human MM, the BHQ880 anti-DKK-1 mAb not only triggered new bone formation but also inhibited MM cell growth.46 A derived clinical trial of the BHQ880 anti-DKK-1 mAb is ongoing. Finally, B cell–activating factor (BAFF) is elevated in the BM plasma of MM patients and mediates osteoclastogenesis. Preclinical studies showing that the anti-BAFF mAb can neutralize this effect and inhibit MM cell growth47 have already translated to a clinical trial showing efficacy in relapsed MM. Most recently, targeting BTK has been shown to block osteoclast formation and growth and MM cell growth in preclinical models, and a related clinical trial will begin soon. These studies illustrate the principle that agents targeting cytokines or accessory cells in the tumor microenvironment can also affect tumor growth, further validating the utility of evaluating MM in the context of its BM milieu.
Development of immune-based therapies
The success of any immune-based strategy may depend on correcting the decreased help, increased suppression, pro-MM growth cytokines, and dysregulated immune-homeostasis that are hallmarks of MM. For example, our studies show that plasmacytoid dendritic cells in MM do not induce immune effector cells and instead promote tumor growth, survival, and drug resistance.48 Maturation of plasmacytoid dendritic cells with CpG oligonucleotides both restored immune function and abrogated their tumor-promoting activity in preclinical models, setting the stage for a derived clinical trial. A second example is the use of anti–IL-17 mAb therapy to target increased TH-17 cytokines promoting MM cell growth in MM.49
There has been long-term interest in immune-based therapies in MM, including mAbs and immunotoxins targeting MM cells and cytokines and novel vaccine strategies. For example, CS-1 is a natural killer cell antigen that is highly and uniformly expressed at the gene and protein level in MM cells, and targeting this antigen with the anti-CS1 mAb elotuzumab triggered MM cytotoxicity in preclinical models of MM in the BM milieu.50 A derived clinical trial of elotuzumab in relapsed refractory MM achieved stable disease but not responses; however, preclinical studies showing that lenalidomide enhances antibody-dependent cellular cytotoxicity of elotuzumab provided the rationale for an ongoing clinical trial of the combination. Therefore, bedside to bench and back studies helped to inform clinical trial strategy for new drug approval. Clinical trials of the CD38 antigen, which is expressed on all MM cells,51 are also ongoing. The maytansinoid toxin has been conjugated to anti-CD138 mAb (CD138-DM), and promising data in vitro and in xenograft models of human MM in mice have provided the framework for a clinical trial of this immunotoxin.52 Finally, either whole-cell or peptide vaccine strategies are undergoing clinical evaluation in MM. For example, vaccination with fusions of dendritic cells with tumor cells allows for the generation of T cell– and humoral tumor–specific host immunity in preclinical models of murine53 and human MM.54 Derived clinical trials of MM-DC fusion vaccinations are achieving high rates of complete response posttransplantation. An alternative promising strategy is the use of peptides for vaccination. For example, CS-1, XBP-1, and CD 138 are functionally significant targets in MM cells,55 and a clinical trial is now ongoing that is vaccinating patients with pooled peptides derived from these antigens, which are predicted to be presented and trigger immunity in patients of particular HLA types. Genomics is being used to define the biologically relevant targets and genetics performed to derive the peptides most likely to induce a host immune response.
Development of next-generation single-agent and combination targeted therapies
Functional oncogenomics is now being used not only for new target discovery and validation of targeted therapies, but also to inform the design of novel single-agent and combination therapies. For example, cyclin D abnormalities are a hallmark of MM, and as a consequence, cyclin D kinase inhibitors, alone and with bortezomib, are now undergoing evaluation in clinical trials.56 mTOR inhibitors have also been combined with bortezomib57 and with lenalidomide58 in clinical trials, based upon synergistic MM cytotoxicity of these combinations in preclinical models. Bortezomib inhibits DNA damage repair in vitro, providing the rationale for its combination with DNA-damaging agents to overcome drug resistance. A randomized phase 3 clinical trial of bortezomib versus bortezomib with pegylated doxorubicin in relapsed/refractory MM showed prolonged progression-free and overall survival rates and increased extent and frequency of response,59 leading to the FDA approval of this combination. Heat shock protein 27 (hsp 27) is increased at a transcript and protein level in bortezomib-refractory patient MM cells; conversely, the p38MAPK inhibitor decreases downstream hsp27 and overcomes bortezomib resistance in MM cell lines and patient cells,60 providing the rationale for a combination clinical trial. Bortezomib also triggers activation of Akt, which is blocked by the Akt inhibitor perifosine. This combination was shown to overcome resistance to bortezomib in preclinical models,61 and phase 1/2 trials of combination therapy showed durable responses, even in the setting of bortezomib resistance.62 A phase 3 clinical trial of bortezomib versus bortezomib with perifosine in relapsed MM is ongoing for FDA approval.
Protein homeostasis represents one of the most attractive novel therapeutic targets in MM. Specifically, inhibition of the proteasome up-regulates aggresomal degradation of protein and, conversely, blockade of aggresomal degradation induces compensatory up-regulation of proteasomal activity.63 Most importantly, blockade of aggresomal and proteasomal degradation of proteins by histone deacetylase (HDAC) inhibitors (eg, vorinostat, panobinostat, and tubacin) and proteasome inhibitors (eg, bortezomib and carfilzomib), respectively, has triggered synergistic MM-cell cytotoxicity in preclinical studies.63–65 International phase 1/2 clinical trials combining the HDAC inhibitors vorinostat or panobinostat with bortezomib have achieved responses in the majority of patients with relapsed bortezomib-refractory MM, and phase 3 clinical trials for FDA registration of these combinations are currently ongoing. An HDAC6 selective inhibitor has rapidly advanced from our laboratory to clinical trials and offers the opportunity to more selectively and potently block aggresomal degradation, alone or combined with bortezomib. It may therefore achieve clinical efficacy without the side-effect profile of fatigue, diarrhea, thrombocytopenia, and cardiac abnormalities attendant to broad type 1 and 2 HDAC inhibitors.
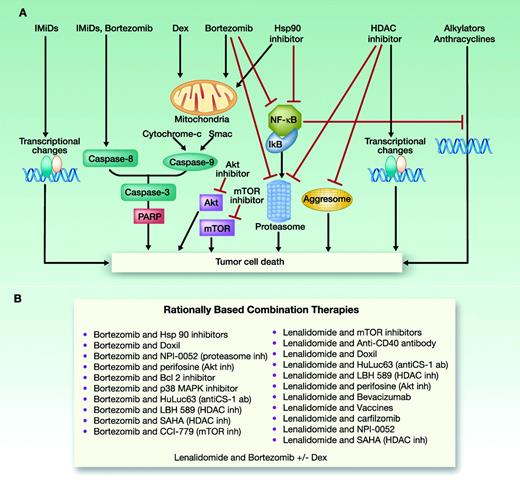
A most exciting combination clinical trial is derived from preclinical studies demonstrating synergistic cytotoxicity induced by combined lenalidomide (caspase 8–mediated apoptosis) and bortezomib (caspase 9–mediated apoptosis) in models of MM cells in the BM milieu. Treatment with lenalidomide, bortezomib, and dexamethasone (RVD) achieved 58% responses in relapsed refractory MM, often refractory to either agent alone.66 Most importantly, RVD combination therapy of newly diagnosed MM achieves 100% responses, with 74% being at least very good partial responses and 52% complete or near complete responses.38 Given these results, a clinical trial is now evaluating whether high-dose therapy and stem-cell transplantation adds value in the context of this high extent and frequency of response to RVD. Recent trials have also compared other 3-drug regimens of cyclophosphamide, bortezomib, and dexamethasone (CyBorD) and a 4-drug regimen (CyRVD). To date, 3-drug regimens have been shown to be optimal and even to achieve molecular complete responses in some cases.67 Therefore, the integration of combination novel agent therapy, predicated upon scientific rationale, is transforming the treatment paradigm in MM (Figure 3).
Rationally based combination treatment in MM. (Modified with permission from Lonial et al.68 )
Rationally based combination treatment in MM. (Modified with permission from Lonial et al.68 )
Summary and future directions
Ongoing and future research is directed at using genomics to develop improved classification and personalized therapy, immune-based therapies, next-generation agents targeting the tumor cell in its microenvironment, and rationally based combination targeted therapies. With the continued rapid evolution of progress in this field, MM will become a chronic illness having sustained complete responses in a significant number of patients.
Disclosures
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Celgene, Bristol Myers Squibb, Merck, Onyx, Millennium, Novartis, and Acetylon. Off-label drug use: None disclosed.
Correspondence
Kenneth C. Anderson, MD, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 557, Boston, MA 02215; Phone: (617) 632-2144; Fax: (617) 632-2140; e-mail: kenneth_anderson@dfci.harvard.edu.