Abstract
Elucidation of the pathogenesis of chronic myeloid leukemia (CML) and the introduction of tyrosine kinase inhibitors (TKIs) has transformed this disease from being invariably fatal to being the type of leukemia with the best prognosis. Median survival associated with CML is estimated at > 20 years. Nevertheless, blast crisis occurs at an incidence of 1%-2% per year, and once this has occurred, treatment options are limited and survival is short. Due to the overall therapeutic success, the prevalence of CML is gradually increasing. The optimal management of this disease includes access to modern therapies and standardized surveillance methods for all patients, which will certainly create challenges. Furthermore, all available TKIs show mild but frequent side effects that may require symptomatic therapy. Adherence to therapy is the key prerequisite for efficacy of the drugs and for long-term success. Comprehensive information on the nature of the disease and the need for the continuous treatment using the appropriate dosages and timely information on efficacy data are key factors for optimal compliance. Standardized laboratory methods are required to provide optimal surveillance according to current recommendations. CML occurs in all age groups. Despite a median age of 55-60 years, particular challenges are the management of the disease in children, young women with the wish to get pregnant, and older patients. The main challenges in the long-term management of CML patients are discussed in this review.
Introduction
With the advent of first- and second-generation tyrosine kinase inhibitors (TKIs), therapy for patients with chronic myeloid leukemia (CML) has grown in complexity. Optimal management of patients on therapy requires exact knowledge of response milestones and of potential toxicities, including approaches to preventing and managing side effects. Imatinib, dasatinib, and nilotinib each have specific considerations regarding safety and toxicity, in addition to a limited number common to this class of ABL kinase inhibitors. The availability of several approved treatment options demands the incorporation of toxicity considerations into the decision making process when choosing among these agents.
CML constitutes about 15% of all leukemia and occurs with an incidence of approximately 1-1.5 in 100 000/year. With an estimated survival rate of 90% at 5 years and an annual mortality rate of 2%,1 the prevalence of CML in 20 years may become 1 in 1000 inhabitants in countries using TKIs for all new patients. This may eventually reach a plateau when the number of newly diagnosed patients equals the number of patients dying with or after CML. However, because CML is still a comparatively rare disease, basic research and treatment evaluation demand national and international collaboration. In daily clinical practice, some CML management areas are still not in line with the current recommendations. Problematic topics are suboptimal timing of treatment decisions under monitoring and unawareness of modern monitoring procedures and new treatment options.
Harmonized surveillance strategies
Although most CML patients treated with imatinib have an excellent response, monitoring through hematological, cytogenetic, and molecular testing is recommended by the European LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN) to promptly identify and optimize treatment for the minority of patients who respond slowly.2,3 Cytogenetics, performed with chromosome banding analysis of BM cell metaphases, is required at 3 and 6 months and then every 6 months until a complete cytogenetic response (CCyR) has been achieved and confirmed. After that, this should be performed every 12 months if regular molecular monitoring cannot be ensured, and always in instances of myelodysplastic features, suboptimal response, or failure. Cytogenetic analysis from BM metaphases is preferred to interphase FISH. However, once a CCyR has been achieved or BM cells cannot be sampled or analyzed in an appropriate number, interphase FISH of blood cells can be used to monitor the completeness of cytogenetic response using BCR-ABL extra-signal, dual-color, or dual-fusion probes and by scoring at least 200 nuclei.2
Because most patients with CML treated with imatinib achieve CCyR, the measurement of residual disease through sensitive molecular methods such as quantification of BCR-ABL transcript levels and real-time quantitative PCR (RQ-PCR) has become particularly important for evaluating treatment success in CML. The ELN and NCCN recommendations advocate monitoring for molecular response every 3-6 months once CCyR has been achieved.2,3 This allows primary and acquired resistances to TKIs to be identified early and for treatment to be adjusted accordingly.2,4 Suboptimal responders and patients with warning features may require more frequent monitoring. Monitoring the response to other TKIs requires the same tests, but earlier and more frequent testing may be appropriate because responses are more rapid.5,6
A key marker of molecular response is the so-called major molecular response (MMR), originally classified as a reduction in BCR-ABL transcripts by at least 3 logs below a standardized baseline value. Achievement of MMR is associated with improved probability of long-term response and improved progression-free survival in patients treated with imatinib.2,7 The German CML Study IV demonstrated a survival advantage for patients achieving MMR after 12 months.8 Despite the proven prognostic significance of MMR, wide variations in the methods used to quantify BCR-ABL and the lack of widely accepted standards have led to considerable variations in results, making comparability between different laboratories difficult. Standardized reporting of BCR-ABL measurements is needed for optimal clinical management, as well as comparison of measurements from different study groups and pooling of results from different studies. An international program is now under way to harmonize the reporting of results according to an international scale. Laboratory-specific conversion factors are only valid for particular instruments and particular standard operating procedures; any change in laboratory protocols or upgrade of equipment will necessitate recalculation of the conversion factor.9
To help improve the comparability of results between centers, accredited reference reagents were developed that are directly linked to the BCR-ABL international scale. The development of these reagents is a significant milestone in the standardization of this clinically important test, but because they are a limited resource, their availability is restricted to manufacturers of secondary reference materials.10 Automated assays in development achieve similar interlaboratory reproducibility to highly standardized nonautomated assays. A short delay (≤ 6 hours) between sampling and blood lysis had a positive impact on interlaboratory reproducibility. Reporting automated BCR-ABL ratios on the international scale is possible using a specific conversion factor that may vary with batches. The Xpert BCR-ABL monitor (Cepheid) assay could be used in a near-patient setting for routine quantification of e13a2 and e14a2 BCR-ABL transcripts, preferably in cooperation with a regional reference laboratory. However, its prognostic impact relative to nonautomated quantification remains to be tested prospectively within appropriate clinical trials.11
Management of treatment-related adverse effects
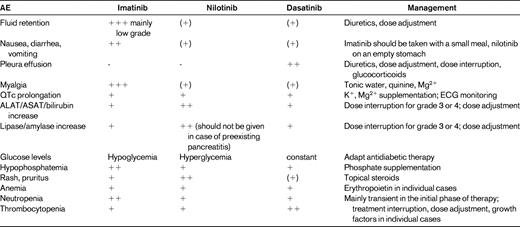
The prevention, detection, and treatment of adverse effects to TKIs is a major factor in improving patient quality of life during therapy and in ensuring adherence to long-term therapy (Table 1). In the International Randomized Study of Interferon versus STI571 (IRIS) study, 4.9% of patients discontinued first-line imatinib because of adverse effects (AEs) during 5 years of follow-up.1 Higher doses of imatinib are associated with higher rates of toxicity, which lead to dose reductions and discontinuations due to AEs.12 Toxicity-adjusted dosage may help to overcome AEs and to improve efficacy with higher dosages.8
Before the introduction of second-generation BCR-ABL inhibitors, treatment options for imatinib-intolerant patients comprised stem cell transplantation, which was associated with significant morbidity and mortality, and Interferon (IFN)-α combined with chemotherapy, which has proven to have significantly inferior efficacy to imatinib. Consequently, the comparatively milder AEs experienced with imatinib were initially better accepted by patients and physicians and symptomatic management of AEs had a major role in patient care. Today, patients experiencing significant AEs on imatinib can be switched to dasatinib or nilotinib, so there is less need or willingness to accept imatinib-associated AEs. Intolerance is also seen with nilotinib or dasatinib therapies. Two separate second-line trials in chronic-phase CML (CP-CML) patients have reported results with a minimum follow-up of 24 months. In the first trial, 58% of patients had their nilotinib treatment interrupted and 19% of patients discontinued treatment as a result of AEs.13 In the second trial, after a minimum follow-up of 2 years, the toxicity-related discontinuation rate for dasatinib was 11% and 62% of patients had their treatment interrupted.14 In 2 single-arm first-line phase 2 studies, 7% and 3% of patients had discontinued nilotinib 400 mg twice daily because of side effects after 17 and 24 months of follow-up, respectively.15,16 In a phase 2 trial of dasatinib, 5% of patients had discontinued 100 mg once daily because of side effects after a median of 24 months of follow-up.17
There is rare nonhematologic cross-intolerance between second-generation TKIs and imatinib. In the nilotinib phase 2 trial in patients with CP or accelerated-phase CML, 1 of 75 (1%) patients with nonhematologic imatinib intolerance experienced a similar grade 3/4 AE, and only 3 of 75 (4%) experienced a similar persistent grade 2 nonhematologic AE on nilotinib. Only 7 of 40 (18%) patients with hematologic imatinib intolerance discontinued nilotinib, all due to grade 3/4 thrombocytopenia.18 In a dasatinib trial in patients with CP-CML, 6 of 46 (13%) patients had hematological cross-intolerance on dasatinib 100 mg once daily and subsequently discontinued dasatinib.19 However, nonhematological cross-intolerance to imatinib was uncommon with both nilotinib (2 of 109; 2%) and dasatinib 100 mg once daily (9 of 225; 4%). The incidence and severity of several types of AEs differ between the TKIs, but there are other AEs indicating TKI class effects.
Cytopenias are the most commonly occurring events in patients with CML receiving BCR-ABL inhibitors. After 18 months of follow-up, patients with CP-CML treated with first-line imatinib 400 mg/d in the IRIS study had cumulative rates of grade 3-4 neutropenia and thrombocytopenia of 14% and 8%, respectively.7 Cytopenia occurs at higher rates in patients treated with nilotinib or dasatinib after previous imatinib treatment. However, in studies of second-generation agents administered as first-line treatment in CP-CML, rates of grade 3-4 cytopenia were lower for both nilotinib and dasatinib (neutropenia, 4%-12% and 21%; thrombocytopenia, 2%-12% and 10%-19%, respectively). Cytopenia during BCR-ABL inhibitor therapy is thought to reflect mainly a reduced reserve of residual nonleukemic BM progenitors that are insufficient to reconstitute peripheral blood counts, rather than a toxicity toward normal hematopoietic cells. It is, however, possible that TKIs might suppress normal hematopoietic stem cell function.
Most cytopenias occur during the first few months of treatment, are self-limiting, and are mild to moderate in severity. The management of cytopenias is described below.20
The intensity of therapy should match the aggressiveness of the disease. That is, in high-risk patients, second-line therapy after treatment failure or advanced disease blood product and growth factor support should be the preferred option over dose reduction or interruption. Conversely, in good-risk patients, transient treatment interruptions or dose reductions are the initial option.
Dose reductions are not indicated for grade 1 or 2 myelosuppression.
Acute dose adjustments are indicated for neutropenia and thrombocytopenia, but not anemia, whereas chronic anemia may require dose reductions.
Myeloid growth factors and erythropoiesis-stimulating agents are important tools for the management of myelosuppression, although they are not labeled for this indication.
All 3 clinically available BCR-ABL inhibitors are associated with fluid retention events, but incidences and clinical presentations differ. In imatinib-treated patients with CP-CML, a fluid retention AE of any type was observed in 62% of patients, including 60% who developed superficial edema and 7% who had other fluid retention AEs such as pleural effusion, ascites, pulmonary edema, or pericardial effusion. However, severe fluid retention AEs were relatively uncommon, with grade 3-4 events occurring in < 1% of patients.1 In the phase 3 study of nilotinib versus imatinib as a first-line treatment, patients receiving imatinib were more likely to experience all-grade peripheral edema, eyelid edema, and periorbital edema (14%, 13%, and 12%, respectively) than patients receiving nilotinib 300 mg twice daily (5%, 1%, and < 1%, respectively) or nilotinib 400 mg twice daily (5%, 2%, and 1%, respectively).5
After 2 years of follow-up with dasatinib 100 mg once daily in patients with CP-CML and previous imatinib treatment, drug-related superficial edema of any grade occurred in 17% (0% grade 3-4), pleural effusion in 14% (2% grade 3, 0% grade 4), and pericardial effusion in 2% of patients (1% grade 3-4).14 In the phase 3 comparison of dasatinib and imatinib as first-line CML treatment, pleural effusions were observed in 26 of 259 (10%) patients receiving dasatinib. No pleural effusion was seen in patients receiving imatinib.6 The origin of dasatinib-associated pleural effusion remains unclear. It has been suggested that inhibition of platelet-derived growth factor receptors or expansion of cytotoxic T-cell and natural killer–cell populations are involved. Dasatinib has been linked to a clonal expansion of T cells and natural killer cells, which is associated with a favorable response.21
The incidence of severe fluid retention AEs is low, but significant and regular monitoring is needed. With imatinib, patients older than 65 years and those with cardiac disease or renal insufficiency are more likely to experience fluid retention.1 With dasatinib treatment in patients who previously received imatinib, various risk factors for pleural effusion have been identified and include older age, pre-existing cardiac disease, hypertension, hypercholesterolemia, autoimmune disease, or skin rash. In addition, pleural effusion occurs more frequently in patients treated with dasatinib in advanced versus CP-CML, and was more common in patients who received the original twice-daily dosing versus current once-daily dosing.14
All patients should be monitored closely for symptoms suggestive of fluid retention, such as dyspnea or dry cough. Evidence of peripheral edema or rapid weight gain indicates that diuretic therapy should be initiated or increased. In severe cases, TKI treatment should be suspended until the edema is controlled, with the dosage reduced at the restart of therapy. Pleural effusion is manageable through dose interruption/reduction and supportive measures, including diuretics and steroids. Considering the short half-life of dasatinib, introducing a weekend holiday (5 days/week schedule) to allow recovery from the off-target activity has been investigated in an attempt to reduce side effects.22
Cardiotoxicity is a rare but potentially serious complication of therapy with all BCR-ABL inhibitors.23 In retrospective analyses, the estimated frequency of congestive heart failure or left ventricular dysfunction during imatinib therapy for CML was 0.5%-1.1%.24 QT prolongation has been noted during second-line treatment with both nilotinib and dasatinib, although the total incidence of QTcF of > 500 msec was < 1% for each agent.5,6 During the nilotinib phase 2 trial program, sudden death occurred in 0.6% of patients, with a similar incidence recorded in an expanded-access program. Contraindications for nilotinib therapy include hypokalemia, hypomagnesemia, or long QT syndrome. Because taking nilotinib with food may lead to increased absorption in the presence of fat, resulting in peak levels increasing the risk of QT prolongation, no food should be consumed 2 hours before and 1 hour after each dose. TKIs should be administered with caution to patients who are deemed at risk of developing prolongation of QTc. Because of the potential consequences of QT prolongation or cardiac events, electrolyte abnormalities should be corrected before both nilotinib and dasatinib therapy. During BCR-ABL inhibitor treatment, strong CYP3A4 inhibitors should be used with caution (imatinib) or avoided (nilotinib and dasatinib), because pharmacokinetic interaction will lead to higher peak and trough levels of the TKI that may be associated with QT prolongation.
In CP-CML patients on first-line imatinib therapy, grade 3-4 elevations in aminotransferases and bilirubin occurred in 5% and 1% of patients, respectively.1 Increased liver enzymes are the most frequent cause of treatment interruption in patients receiving first-line nilotinib therapy (13 of 61 patients; 21%).5 During dasatinib treatment of CP-CML after imatinib failure or as a first-line treatment, grade 3/4 elevated aminotransferases or bilirubin are rare and occurred in < 1% of patients.6 In the nilotinib phase 2 study in CP-CML patients after imatinib failure, grade 3-4 serum lipase elevation was reported in 18% and grade 3-4 hyperglycemia in 12% of patients.13 In most cases, this was self-limiting, but pancreatitis was reported in 1% of patients. With first-line nilotinib treatment, rates of grade 3-4 lipase elevation and hyperglycemia seemed to be lower (3-8 and 3%, respectively).5,15,16 Management of metabolic side effects include adjustment of treatment of diabetes mellitus (imatinib: reduced intensity, nilotinib: increased intensity), treatment interruption in case of increased activity of lipase, and omitting nilotinib after a history of acute pancreatitis. Liver function tests should be done before therapy. Sole hyperbilirubinemia at the start of TKI therapy is often self-limiting. Polymorphisms in the gene coding for uridine diphosphate glucuronosyltransferase 1A1 (UDG1A1), which are associated with Gilbert syndrome, predict for susceptibility to nilotinib-induced hyperbilirubinemia. TKI therapy should be interrupted in case of grade 3 or 4 liver toxicity, and treatment should be resumed at a lower dose. Gradual adjustment to the initial dose is recommended if possible. Long-term management of CML may include the management of other diseases that appear independently. Pharmacokinetic interactions should be considered in such cases. Irinotecan exposure, for example, as administered for colorectal cancer, should be avoided in case of nilotinib therapy due to the inhibition of UDG1A1 by nilotinib with the consecutive accumulation of irinotecan.
Altered mineral metabolism occurs with all BCR-ABL inhibitors. Correction of electrolytes by supplementation is recommended due to their influence on cardiac function. Hypophosphatemia is common during imatinib therapy, occurring in approximately 50% of patients.1 In patients with CP-CML who received either dasatinib or nilotinib, grade 3-4 hypophosphatemia was seen in 10%.5,6 Hypophosphatemia is part of altered bone homeostasis and phosphate may be supplemented. There are currently no specific monitoring recommendations for changes in bone density. Monitoring for osteoporosis in older patients and periodic electrolyte studies are part of routine toxicity screening and are probably sufficient to allow the detection of cases in which imbalances develop and further workup is required.4,20
Low-grade gastrointestinal disturbances were observed in approximately 25%-50% of patients on imatinib, although the incidence of grade 3-4 events was low (0%-3%).5–8,13–17 The incidence of such low-grade symptoms has improved significantly with dasatinib or nilotinib therapy.
During first-line imatinib treatment of CP-CML, rash was noted in 34% of patients and pruritus in 7%.1 After imatinib treatment, rash and pruritus occurred with nilotinib in 31% and 26% of CP-CML patients, respectively,13 and with dasatinib in 17% and 10%, respectively.14 In the ENESTnd study, all-grade pruritus and rash was observed in 5% and 11% of patients receiving imatinib, and 13%-15% and 31%-36% of patients receiving nilotinib 300 or 400 mg twice daily, respectively.5 In the DASISION study, all-grade rash was reported in 17% and 11% of patients, respectively.6 These reactions are nonallergic and self-limiting and can be managed with antihistamines or topical steroids.
Musculoskeletal pain and muscle cramps were reported by nearly 50% of patients on imatinib.1 However, these AEs seem to be less problematic with second-generation agents. In the first-line setting for patients with CP-CML, any-grade muscle spasm was experienced by 24% of patients receiving imatinib, 7% of patients receiving nilotinib 300 mg twice daily, and 6% of those receiving nilotinib 400 mg twice daily. Incidences of any-grade myalgia were 10% in all arms.5 In the phase 3 study of first-line dasatinib and imatinib, incidences were higher in the imatinib arm for any-grade myalgia (12% vs 6%).6 Symptomatic relief from muscle cramps can frequently be achieved with calcium and magnesium supplements or quinine.
Dasatinib has been associated with bleeding-related events, mostly related to severe thrombocytopenia. In the phase 3 study of first-line dasatinib and imatinib in patients with CP-CML, all-grade bleeding events were found in 5% of patients in both arms; grade 3-4 events were experienced by 1 of 259 patients in the dasatinib arm, and by 2 of 260 patients in the imatinib arm (< 1% in both cases).6 In vitro studies have suggested that TKIs, particularly dasatinib, have various inhibitory effects against platelets.25
Proposed definition of intolerance
A patient has TKI intolerance if one or more of the following criteria have been met: (1) any life-threatening grade 4 nonhematological toxicity; (2) any grade 3/4 nonhematological toxicity that has recurred despite dose reduction; (3) any grade 2 nonhematological toxicity that persists for more than a month despite optimal supportive measures; or (4) grade 3-4 hematological toxicity that is unresponsive to supportive measures and would require dose reductions below the accepted minimal effective dose.26
Beyond these clear-cut definitions of intolerance, there is a gray area governed as much by objective findings as by the availability of alternatives that may offer a better quality of life. Therefore, in addition to the criteria proposed, intolerance could also be defined as any combination of nonhematological toxicities of any grade that persist despite optimal supportive measures and compromise quality of life to such an extent that a change of therapy is justified.26
Conception and pregnancy
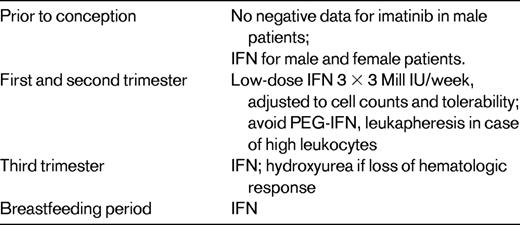
With the improved survival rates offered by the TKIs has come the necessity of addressing issues relating to fertility and parenting. Children fathered by men taking imatinib at the time of conception appear healthy and current advice is not to discontinue treatment. In contrast, the data relating to children born to women exposed to imatinib during pregnancy are less encouraging. Although numbers are small, there has been a disturbing cluster of rare congenital malformations such that imatinib cannot be safely recommended, particularly during the period of organogenesis.27 There is limited information regarding alternatives in the successful management of CML during pregnancy. Most of these data are from case reports using leukapheresis, hydroxyurea in the third trimester, and low-dose IFN-α to maintain the response.28 Pegylated IFN-α must not be used in pregnancy due to accumulation of polyethylene glycol. Despite individual case reports in the literature, information on the outcome of pregnancies after nilotinib and dasatinib therapy is limited. Larger series indicate that an adequate response after restarting imatinib after discontinuation in pregnancy is seen only in patients who had an optimal response (MMR) before stopping the drug. Therefore, women desiring to get pregnant, at least an MMR should be achieved to reduce the risk of treatment failure after the reintroduction of therapy.29 TKI therapy should be discontinued at least 3 months before conception in both parents. RQ-PCR analyses from peripheral blood in 6-weekly intervals are useful to check for loss of molecular response (Table 2).
CML in older patients and pharmacologic interaction
Reports of clinical studies underestimate the true age of the CML population, which is ∼ 60 years. Elderly patients might have less of a chance to be included in trials and thus have reduced access to investigational therapies. Median age differs between cancer registries and clinical trials by 10-20 years.30
The age of CML patients is considered an important prognostic factor and is therefore included in both the Sokal and EURO risk scores. TKIs are prescribed for prolonged periods, often in patients with comorbidities. The use of additional drugs is therefore more frequent in older patients and may create pharmacologic interaction with TKIs. The Italian CML group analyzed the relationship between age and outcome in 559 early CP-CML patients treated with frontline imatinib, with a median follow-up of 60 months; 115 patients (21%) were older than 65 years. The complete cytogenetic and MMR rates were similar in the 2 age groups. In older patients, event-free survival (55% vs 67%), failure-free survival (78% vs 92%), progression-free survival (62% vs 78%), and overall survival (75% vs 89%) were significantly inferior due to a higher proportion of deaths occurred in complete hematologic response and therefore unrelated to CML progression (15% vs 3%, P < .0001). The outcome was similar once those deaths were censored, indicating that response to imatinib is not affected by age.31
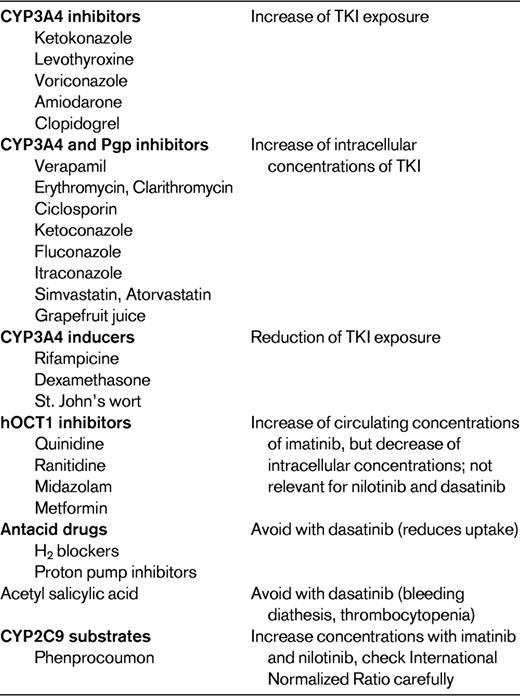
Pharmacokinetic drug interactions and safety recommendations are best characterized for imatinib. The other TKIs, which have just recently been marketed, have so far only a limited documentation about clinically relevant interactions. Their concentration profile might be affected to a more dramatic degree by interactions than imatinib exposure. The 3 TKIs reviewed herein are indeed substrates of several drug transporters and metabolizing enzymes. They are also capable of inhibiting drug transporters and enzymes, making their disposition and metabolism rather complex and difficult to predict. The results from pharmacokinetic studies must be translated into treatment adjustment recommendations for the clinical oncology practice, where these drugs are administered on a daily basis in patients receiving various comedications. The actual relevance of predicted drug interactions is thus still uncertain. Therapeutic drug monitoring of TKIs should be considered if a drug interaction is suspected or in cases of toxicity or lack of satisfactory clinical response.
The available evidence and pharmacologic mechanisms of interactions between imatinib, dasatinib, and nilotinib and widely prescribed comedications, including known inhibitors or inducers of cytochromes P450 or drug transporters, is summarized in Table 3. Interactions should therefore be considered when administering inhibitors of the CYP3A family in combination with imatinib. Strong inhibition, such as achieved with ketoconazole, caused a 40% increase of imatinib exposure in healthy volunteers.30 Interactions are likely to occur with other inhibitors of CYP3A4, such as levothyroxine, voriconazole, or amiodarone, leading to an increase in plasma concentrations of imatinib. Concomitant administration of imatinib with inhibitors of both CYP3A4 and Pgp increase not only plasma but also intracellular imatinib concentrations. Dual CYP3A4 and Pgp inhibitors such as verapamil, erythromycin, clarithromycin, ciclosporin, ketoconazole, fluconazole, and itraconazole increase intracellular concentrations of imatinib by inhibiting both its metabolism and its efflux by Pgp and might therefore increase its cellular toxicity.
Concomitant administration of CYP3A4 inducers such as rifampicin or certain antiepileptics may lead to a reduction of as much as 74% in imatinib exposure. Moreover, the pharmacokinetic profile of imatinib was significantly altered by St John's wort, with reductions of 30% in the median area under the concentration-time curve. Interactions with quinidine, ranitidine, or midazolam, known inhibitors of hOCT1, may paradoxically increase the circulating concentrations of imatinib but decrease the intracellular exposure of target cancer cells. TKIs can also inhibit drug transporters and enzymes, leading to changes in the exposure of coadministered drugs. Imatinib enhances the intestinal absorption of ciclosporin, a CYP3A4, and Pgp substrate, and may increase the pharmacologic effects and possibly toxicity of ciclosporin. Moreover, the clearance of simvastatin (a CYP3A4 substrate) was reduced by 70% when associated with imatinib. Administration of imatinib together with metoprolol, a CYP2D6 substrate, resulted in an increase in metoprolol exposure of 23%.32
Quality of life
With increasing survival and therapy options, the need for implementation of health-related quality of life (HRQoL) is obvious. In an extensive overview study, the state of HRQoL studies concerning leukemia patients was elaborated.33 Since 2000, only 6 prospective studies in leukemias were conducted. HRQoL is a multidimensional concept representing the physiological, psychological, and social influences of the disease and the therapeutic process from the patient's perspective. After systematic review of published studies, there is clear evidence that imatinib provides an advantage in terms of HRQoL over IFN-based treatments. Documenting HRQoL and side effects of novel CML treatments from the patient's perspective is needed to evaluate the overall treatment effectiveness and net clinical benefit of newer therapeutic strategies.34
Adherence to therapy
Nonadherence is a known problem among patients with chronic disease receiving long-term medication.35 With imatinib treatment, an analysis of patient-level pharmacy claims data in 4043 imatinib-treated patients with CML or gastrointestinal stromal tumors found that patients with CML took an average of 78% of their prescribed imatinib therapy during the 24-month study period. An analysis of pharmacy claims revealed that the adherence rate to imatinib at doses of 400 to < 600 mg/d was 66%, which decreased to 52% for patients prescribed 600 to < 800 mg/d. Nonadherence to treatment is likely to lead to reduced trough drug concentrations, which are associated with reduced efficacy. Furthermore, studies have shown that patients with CML who have lower levels of adherence to imatinib treatment have significantly reduced treatment responses.36,37
In a study performed in the United Kingdom, 87 patients with CP-CML treated with imatinib 400 mg/d for a median of 59.7 months (range, 25-104 months) had adherence monitored during a 3-month period within CCyR using a microelectronic monitoring device. Twenty-three patients (26.4%) had adherence of < 90%; in 12 of these patients (14%), adherence was < 80%. There was a strong correlation between adherence rate and the 6-year probability of MMR (28.4% vs 94.5%) and CMR (0% vs 43.8%). Multivariate analysis identified adherence and expression of the molecular human OCT-1 as the only independent predictors for MMR.38 Further, poor adherence is the principal factor contributing to the loss of cytogenetic responses and treatment failure in patients on long-term therapy.39
Patient education regarding adherence to TKI therapy and close monitoring of adherence is critical to achieve optimal responses. Drug-delivery devices with reminder function, diaries, or text-messaging reminders may help to increase adherence. However, a successful option to increase adherence to therapy are regular (ie, 3-6 monthly) assessments of the molecular response by RQ-PCR with transfer of the results to the patient, including interpretation, at earliest convenience. Drug-level monitoring without informing the patient is not recommended. Physicians and pharmacists should educate patients and closely monitor adherence to therapy, because improving adherence and limiting treatment interruptions may not only optimize clinical outcomes, but may also reduce the economic burden of CML.
Pharmacoeconomics
Access to medicines in developing countries continues to be a significant problem due to lack of insurance and lack of affordability. The Glivec International Patient Assistance Program (GIPAP) is a project that provides free treatment to eligible CML patients in 80 countries worldwide. Data for 13 568 patients across 15 countries were analyzed over the 2005-2007 period. Having controlled for age, location, and occupation, the analysis showed that patients were significantly more likely to move toward a better health state after receiving treatment irrespective of their disease stage at the point of entry to the program. Overall, GIPAP sets a good example for access to treatment in developing countries, where such programs can substitute or complement local efforts to provide care to eligible patients.40
For pharmacoeconomic analyses of the actual costs of management of CML patients, daily treatment costs, costs of monitoring, duration of therapy, and cost of alternative therapies should be considered. This may allow the acceptance of a more costly second-generation TKI therapy or a combination therapy over imatinib with higher chance for early and permanent discontinuation.
Discontinuation of therapy
Long-term TKI treatment leads to a gradual reduction of residual disease in the majority of patients, and in some patients, no residual disease is detectable with RQ-PCR.5,6 If treatment is discontinued, disease reappears as a rule within a few weeks to months in the majority of patients. Overall, improvement of therapy with increased efficacy will provide the chance of treatment discontinuation. The question of whether TKI treatment of patients in CML should be continued or if it can be interrupted without risk is important because of side effects during long treatment, even if tolerable, and high costs of treatment. Before the imatinib era, a French group published data on the possibility of stopping IFN-α after the achievement of CCyR in 15 patients in CP or accelerated phase at diagnosis.41 Without treatment, 7 of 15 (47%) patients remained in CCyR after a median observation time of 36 months (range, 6-105 months). Persistent CCyR rate without treatment was clearly higher in case of CCyR for at least 2 years before stopping IFN.
The Multicenter STop IMatinib (STIM) trial was started in France to confirm these promising results in a prospective study and to select patients on imatinib for at least 3 years and with stable undetectable BCR-ABL CMR for at least 2 years. One hundred patients were enrolled between 2007 and 2009; 69 patients had at least 12 months of follow-up (median, 24 months; range, 13-30), and 42 (61%) of these 69 patients relapsed (40 before 6 months, 1 patient at month 7, and 1 at month 19). At 12 months, the probability of persistent molecular remission for these 69 patients was 41%. All patients who relapsed responded to reintroduction of imatinib: 16 of the 42 patients who relapsed showed decreases in their BCR-ABL levels and 26 achieved PCR negativity that was sustained after imatinib restart.42
Cooperation with advocacy groups
The CML journey has changed dramatically. More and more patients are being cared for in an ambulatory care setting, where they have limited opportunities for interaction with other patients and health professionals. The value of educating patients in general is often underestimated. Lack of time is a key issue in the ambulatory care setting. The potential of patient groups as a source of information and support should be used more often. International patient groups have managed to reach more patients by involving health professionals in their organizations.
People living with CML want to live their lives as normally as possible, and every effort should be made to ensure that adherence-promoting strategies help to achieve this goal. Nonadherence is a complex phenomenon; health professionals are aware of the impact of nonadherence in the CML setting, but are not aware of the scope of the problem and what they can do to promote adherence. The most important way to promote adherence to oral CML medicines is by talking to patients and providing adequate explanations regarding their disease and their medicines.
Acknowledgments
The cooperation and discussion with colleagues and patient advocates from the international CML community is gratefully acknowledged.
Disclosures
Conflict-of-interest disclosure: The author has received research funding from Novartis, BMS, and Ariad and has been a member of advisory boards for Novartis, BMS, Ariad, and Merck. Off-label drug use: None disclosed.
Correspondence
Prof Dr Andreas Hochhaus, Universitätsklinikum Jena, Klinik für Innere Medizin II, Abteilung Hämatologie und internistische Onkologie, Erlanger Allee 101, 07740 Jena, Germany; Phone: 49-3641-932-4201; Fax: 49-3641-932-4202; e-mail: andreas.hochhaus@med.uni-jena.de.