Abstract
In younger patients with acute myeloid leukemia (AML), initial treatment has provided very good control of the disease. Induction therapy has used combination chemotherapy, with anthracycline and cytarabine as the foundation. Recent trials support dose intensification of anthracycline in induction. Intensive postremission therapy further contributes to improving survival. The addition of targeted therapy with gemtuzumab ozogamicin to standard therapy has not improved on these outcomes. Newer agents targeted to specific molecular abnormalities or survival mechanisms in the leukemic cell are being studied as future additions to the current standard therapy.
Acute myeloid leukemia (AML) is one of the most common types of leukemia in adults.1 In young patients (defined as those ≤ 60 years old), the prognosis is better than for their older counterparts. Despite heterogeneity of the disease, with the exception of acute promyelocytic leukemia, this disease has been treated with a “one size fits all” approach. For almost 40 years, the use of continuous infusion cytarabine combined with another agent, usually an anthracycline or anthracenedione, the “3+7” regimen, has been the mainstay of therapy.2 Response rates for induction with standard chemotherapy have ranged from 50% to 75%. The addition of other drugs3 and intensification of the cytarabine dose4,5 to this approach have failed to improve outcomes. More recent work has attempted to improve patient outcomes by intensifying the doses of anthracyclines or by adding targeted therapies, such as gemtuzumab ozogamicin. Intensive consolidation chemotherapy, consisting of cytarabine with or without other agents, has been employed to maintain the remission and cure the disease. Alternative consolidation therapies using allogeneic hematopoietic cell transplantation (HCT) based on the initial cytogenetic6,7 and molecular studies8 have also been proposed.
Dose intensification approaches in AML have been previously unsuccessful partially because patients developed significant toxicity from prolonged pancytopenia. However, current supportive care practices have made dose intensification feasible. The addition of hematopoietic growth factors has reduced the length of neutropenia and early deaths. Improved broad-spectrum antibiotics and antifungal therapy (particularly the newer azoles) have prevented fatal systemic infections and have reduced the morbidity from supportive therapy. Improvements in transfusion support have also reduced the morbidity from blood products. Table 1 presents current supportive care guidelines for patients undergoing induction/consolidation chemotherapy.
Guidelines for supportive care in AML*

G-CSF indicates granulocyte-colony stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; CMV, cytomegalovirus; PO, define here.
*Adapted from the National Comprehensive Cancer Network Guidelines, version 3, 2010. http://www.nccn.org/professionals/physician_gls/PDF/aml.pdf.
This review will discuss the most recent studies of initial therapy for younger patients with newly diagnosed AML and present targeted agents tailored to the molecular/genetic abnormalities of this heterogeneous disorder.
Initial Therapy in Younger Patients
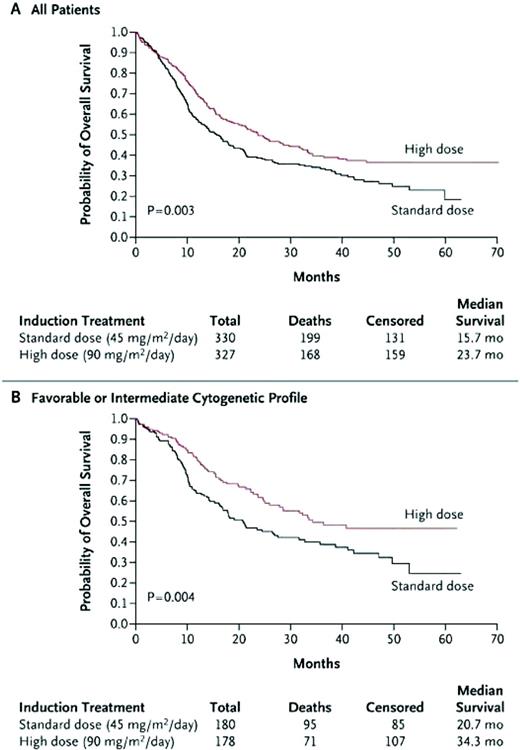
In a Southwest Oncology Group (SWOG) study, younger AML patients who were given a high dose of daunorubicin (70 mg/m2 daily for 3 days) had excellent complete remission (CR) rates.9 The Cancer and Leukemia Group B (CALGB) performed a phase 1/2 study, in which daunorubicin and etoposide were dose escalated, and given with a fixed dose of cytarabine with or without the multidrug resistance modulator PSC-833 (valspodar). This study, which confirmed the safety of intensifying daunorubicin to doses of 95 mg/m2, demonstrated a high induction CR rate.10 This ability to safely intensify daunorubicin led the Eastern Cooperative Oncology Group (ECOG) to study standard-dose cytarabine + daunorubicin at 45 or 90 mg/m2 each for 3 days (E1900 study). This study, which enrolled 647 patients, demonstrated an improved CR rate of 70.6% for the high-dose group, compared with 57.3% for the standard arm. Remission rates were similar or better for the high-dose daunorubicin group across all cytogenetic and molecular subsets. In addition, no increased grade 3/4/5 toxicities or early death rates were shown. Cardiac toxicities—which included dysrhythmias, congestive heart failure, and reduced ejection fractions on preconsolidation and pretransplantation evaluations—occurred infrequently and did not limit the dose intensification strategy, even in patients who received two induction cycles (total 135 mg/m2 of daunorubicin). The anthracycline intensification allowed more patients in the high-dose arm to receive postremission therapy, which consisted of two cycles of high-dose cytarabine, followed by gemtuzumab ozogamicin randomization and autologous HCT. The improved CR rates translated to a significantly higher overall survival (OS) (Figure 1A). Survival was particularly improved for patients with favorable or intermediate cytogenetics (Figure 1B) or in those who were younger than 50 years of age (not shown). Due to the small numbers in some of the groups, the study was unable to demonstrate an improved survival for patients older than 50 years of age or for those patients with unfavorable risk cytogenetics or Fms-like tyrosine kinase 3 (FLT3)-internal tandem duplication or mixed lineage leukemia (MLL)-partial tandem duplication mutations.11 Therefore, dose intensification of an anthracycline should be considered for all patients below the age of 60 years, at least until a better induction regimen is found.
Comparison of standard-dose to high-dose daunorubicin (E1900). (A) All patients—OS. (B) Favorable and intermediate-risk cytogenetic patients—OS.
Comparison of standard-dose to high-dose daunorubicin (E1900). (A) All patients—OS. (B) Favorable and intermediate-risk cytogenetic patients—OS.
The Children's Oncology Group introduced another approach to dose intensification therapy in which a second induction was given at a prescribed time point regardless of response. This timed-sequential therapy was effective in producing CR and improving OS.12 The German AML study group (AMLSG) investigated a similar concept in adults. In this dose escalation study, high-dose cytarabine and mitoxantrone (s-HAM) were given at 83% of the HAM regimen dose, but administered at an interval of 11 to 12 days, as opposed to delaying the therapy until count recovery. Of the 172 patients entered in the trial, 143 (83%) responded, with 62% achieving a CR and 22% achieving CR with incomplete platelet recovery (CRi). Seventy-five patients were able to receive postremission therapy. The OS was impressive, albeit with a short follow-up of only 13 months.13 Although this approach has been described as ”timed sequential” (trying to capture and damage cycling cells), it is not clear whether this method represents anything other than giving intensive consolidation at the time of minimal residual disease.14 A more recent trial by the Acute Leukemia French Association (the ALFA 9000 trial) showed that the “3+7” induction, with an intense dose of daunorubicin at 80 mg/m2 for 3 days and cytarabine 200 mg/m2 for 7 days, could be as effective as double induction or timed-sequential therapy. In that study, all three arms received the same consolidation therapy: amsacrine and cytarabine, followed by mitoxantrone, cytarabine, and etoposide.15 Although the relapse-free survival (RFS) was improved for patients in the timed-sequential group who were younger than 50 years old, the OS was not different among the three regimens. Thus, one could surmise that the simpler “3+7” regimen has outcomes equivalent to the timed-sequential and double induction therapy, but with the advantage of reduced morbidity, length of neutropenia, and days of hospitalization.
Cytarabine intensification in induction has been another approach to attaining initial control of the disease. The standard dose of cytarabine has been 100 or 200 mg/m2 given as a continuous infusion over 7 days.16 However, several groups have attempted to improve survival with high doses of cytarabine (18 to 24 g/m2) given as induction therapy over 4 to 6 days. Although RFS was improved in each of these trials, a recent meta-analysis comparing standard to high-dose cytarabine in induction showed no improvement in OS.17
Finally, for patients who cannot receive anthracyclines because of preexisting cardiac disease, induction therapy with either high-dose cytarabine or a purine analog-containing regimen remains an option. These regimens have comparable remission rates to the “3+7” regimen without worsening cardiac function.
Which Anthracycline Is Best?
Much debate has occurred as to which of the anthracyclines should be used in induction therapy. Idarubicin (4-demethoxydaunorubicin), a more lipophilic congener of daunorubicin with a longer plasma half-life, has been used in combination induction therapy in several large studies with excellent results. Earlier trials that compared cytarabine with idarubicin 12 mg/m2 versus daunorubicin 45 mg/m2 have demonstrated better CR rates or OS for the idarubicin-containing induction therapy.18–20 In retrospect, the results have been clouded by the lack of equipoise in intensity. Later studies by ECOG using daunorubicin 60 mg/m2 for 3 days in induction have noted CR rates similar to those shown with prior idarubicin studies.21
More recently, the European Organisation for Research and Treatment of Cancer leukemia lroup and Gruppo Italiano Malattie Ematologiche dell'Adulto (EORTC/ GIMEMA) randomized 2157 patients (ages 15 to 60 years) with AML to receive intensive induction-consolidation chemotherapy containing either daunorubicin, idarubicin, or mitoxantrone prior to allogeneic or autologous HCT, depending on the availability of a sibling donor. The overall CR rate was similar for both the idarubicin and daunorubicin treatment groups (Table 2). Disease-free survival (DFS) and survival from CR were significantly shorter in the daunorubicin arm; however, OS was similar. The group recommended idarubicin for patients not undergoing allogeneic HCT.22
Comparison of idarubicin and daunorubicin in induction in younger patients
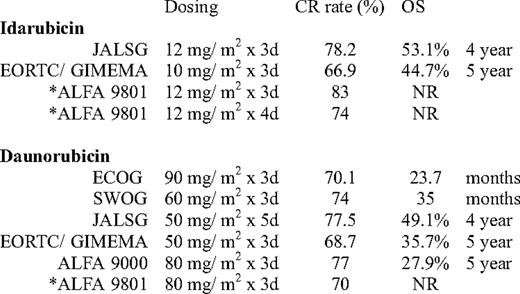
NR indicates not reported.
*Patients age 50–70 years.
Another trial by the ALFA group (ALFA-9801) evaluated dose intensity in patients above the age of 50 years. Patients were randomized to one of three regimens containing cytarabine 200 mg/m2 with either daunorubicin 80 mg/m2 for 3 days, idarubicin 12 mg/m2 for 3 days, or idarubicin 12 mg/m2 for 4 days. The idarubicin-containing regimens had a better CR rate than the daunorubicin regimen, but showed no improvement in event-free survival or OS.23 In this trial and in the aforementioned ECOG- E1900 trial, in patients over age 50 years, the benefit of dose intensification is less clear.
Finally, a recently presented trial from the JALSG (Japan Adult Leukemia Study Group) compared idarubicin with a more intense dose of daunorubicin. In this multicenter randomized trial, 1064 patients with de novo AML (ages 15–64 years) received either daunorubicin 50 mg/m2 for 5 days or idarubicin 12 mg/m2 for 3 days. Consolidation consisted of either high-dose cytarabine or various combinations of chemotherapy with standard-dose cytarabine. The groups were well matched for patient and disease characteristics. There was no difference in CR rates or OS between the two groups. In patients treated with idarubicin, there was a slightly prolonged count recovery and more sepsis and early deaths.24
In summary, the new standard for induction therapy for younger patients with AML should be the “3+7” regimen with daunorubicin at 90 mg/m2 for 3 days and cytarabine 100 mg/m2 for 7 days. Although the substitution of daunorubicin at 60 to 80 mg/m2 or idarubicin 12 mg/m2 in the regimen have produced similar CR rates (Table 2), daunorubicin at 90 mg/m2 has been the only dose to result in an improved OS in a randomized trial. Other drawbacks include a slight delay in count recovery and sepsis if using idarubicin over daunorubicin.22‒24
Gemtuzumab Ozogamicin-Targeted Therapy
The British National Cancer Research Institute, formerly the Medical Research Council, evaluated the addition of gemtuzumab ozogamicin to various combinations of chemotherapy. With the exception of thioguanine-containing regimens, adding gemtuzumab ozogamicin was tolerable.25 The AML 15 trial, which studied the addition of gemtuzumab ozogamicin intensification in 1115 AML patients younger than 60 years of age, demonstrated a CR rate of 85% (CR and CRi). Preliminary analysis showed no difference in CR rates between patients who received the gemtuzumab ozogamicin and those who did not; however, the analysis also noted an improved DFS, in patients with favorable and intermediate-risk cytogenetics who had received gemtuzumab ozogamicin.26 Further follow-up has tempered these positive results, because OS was not improved with the addition of gemtuzumab ozogamicin.27
A recently completed phase III trial from the Southwest Oncology Group (SWOG), S0106, compared cytarabine at 100 mg/m2 continuous infusion for 7 days + daunorubicin 60 mg/m2 for 3 days to the same dose of cytarabine + daunorubicin at 45 mg/m2 for 3 days and gemtuzumab ozogamicin at 6 mg/m2 as a single dose (based on the phase I maximum-tolerated dose)28 on day 4. Demographics were similar between the two groups (n = 627 patients total). Interim results from the trial showed no improvement in CR rates (69% vs 66%, respectively) or OS (31 v 35 months, respectively) between the two groups. Additionally, the study also showed in the induction phase, a significantly higher fatal toxicity rate in patients who received gemtuzumab ozogamicin.29 These disappointing results have prompted the removal of commercially available gemtuzumab ozogamicin from the US market.
Intensive Consolidation
High-dose cytarabine (HIDAC) is now considered the standard of care for consolidation in the younger patient with AML,30 especially for patients with core-binding factor leukemia.31 Multiagent chemotherapy consolidation is not superior to HIDAC.32 Retrospective analysis from CALGB suggests one should treat with a minimum of one cycle of HIDAC and autologous HCT or four cycles of HIDAC for optimal outcomes.33 Because HIDAC is not sufficient to maintain the CR in patients with unfavorable risk disease, allogeneic HCT should be the consolidation of choice.34
In younger patients with AML, normal cytogenetics/intermediate-risk disease comprises over half of the cases. There is significant heterogeneity in the consolidation approach for these patients. The United States Intergroup trial recommended intermediate-risk patients be treated with intensive chemotherapy (HIDAC).21 However, the German AMLSG trial has presented compelling data in patients with cytogenetically normal AML that further risk stratification using molecular makers can delineate a more unfavorable group. Based on this analysis, allogeneic HCT benefited patients who had FLT3 ITD mutation and/or were nucleophosmin-1 and CCAAT/enhancer-binding protein-α gene negative.8 Moreover, a recent meta-analysis demonstrated a relapse and survival advantage for allogeneic HCT over conventional chemotherapy and autologous HCT in patients with intermediate- and unfavorable risk disease in first CR.7 Table 3 lists the current options for initial therapy, including consolidation approaches, based on the cytogenetic and molecular testing results.
The Future of Initial Chemotherapy in AML: Targeted Therapies?
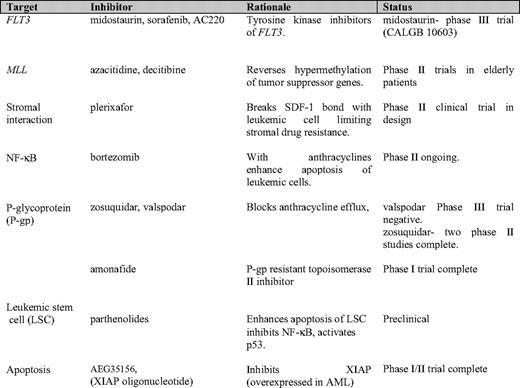
So, how do we improve on the current results? Given that AML is a heterogeneous disease, then the most logical reasoning is to tailor the initial approach to the disease. This has to be done with a rapid return of molecular information at diagnosis that would allow a patient-specific approach. Table 4 illustrates the newer targeted therapies under investigation in younger patients with AML. FLT3 and MLL are known molecular abnormalities in AML that are associated with a worse prognosis. This association persists despite dose intensification. The other presented targets are areas of investigation and interest in AML biology. The next step will be to see whether adding targeted therapies to the current induction chemotherapy helps to induce a higher rate of remissions and/or prolonged survival.
FLT3 ITD and tyrosine kinase domain (TKD) mutations are molecular abnormalities present in 25% to 30% of AML patients. These abnormalities are associated with worse DFS and OS outcomes, regardless of cytogenetic risk.35 Midostaurin is an oral tyrosine kinase inhibitor that has demonstrated efficacy in inhibiting this abnormal gene. A reduction of peripheral circulating blasts was demonstrated in 7 of 20 patients with relapsed FLT3+ AML treated with single-agent PKC 412 (midostaurin).36 An ongoing international intergroup trial led by CALGB (10603 RATIFY [Randomized AML Trial In FLT3 in <60 Year Olds]) aims to determine whether adding midostaurin to daunorubicin and cytarabine in induction, consolidation, and as maintenance improves CR rates and OS. A recent phase I/II trial published from the M. D. Anderson Cancer Center used idarubicin and a higher dose cytarabine with the addition of sorafenib (a multikinase inhibitor) up to 400 mg orally twice daily for 7 days as induction therapy in patients younger than 65 years with relapsed/refractory AML. In that study, very good remission rates were shown, particularly in those who were FLT ITD or TKD+.37 A second-generation FLT3 inhibitor (AC220) has shown more potency and kinase selectivity than the first-generation FLT3 inhibitors. In preclinical mouse models, a dose of 1 mg/kg/day was efficacious.38 Future studies with these agents in patients with de novo FLT3+ AML are warranted, even if the CALGB 10603 trial is negative.
Leukemic cells that harbor the MLL gene enter remission as often as those that do not express the gene; however, the remission duration is usually shorter, resulting in a worse OS. Recently, investigators have suggested that reversing the complex epigenetic process through agents (eg, azacitidine, decitibine, or histone deacetylase [HDAC]) inhibitors, may play a role in the treatment of MLL-expressing leukemia.39
Several drugs have targeted the stromal environment of the bone marrow. Work has focused on the disruption of the stromal–AML cell interaction as a way to improve outcomes.40 Plerixafor disrupts the leukemic cell/stromal–CXCR4/SDF-1 bond, preventing growth of the leukemic cell and inhibiting drug resistance.41,42
Nuclear factor-kappa B (NF-κB) is an integral molecule in the growth and survival of the leukemic cell. The Hematology/Oncology Group at The Massachusetts General Hospital has embarked on the study of its inhibitor, bortezomib, in combination with idarubicin and cytarabine in relapsed patients and in newly diagnosed patients older than 60 years of age. The maximum dose of 1.5 mg/m2—given on days 1, 4, 8 and 11 of induction—was well tolerated43 and will be studied in a phase II trial. The X-linked inhibitor of apoptotic proteins (XIAP) confers chemoresistance in AML cell lines. Inhibition of this protein with AEG35156, an XIAP antisense oligonucleotide reduces XIAP mRNA. In a phase I/II trial, a CR was attained in 47% (15/32) of patients with relapsed/refractory AML treated with the highest planned dose of 350 mg/m2 daily on days 1–3 and day 8.44 A randomized phase II trial is planned.
The use of a P-glycoprotein (P-gp) inhibitor has held promise in the treatment of myeloid leukemia. However, a recently completed randomized trial by CALGB, in AML patients younger than age 60 years, noted that the addition of valspodar (PSC-833) to cytarabine, a dose-intensified daunorubicin and etoposide did not improve remission rates or OS. Furthermore, there was increased grade 3/4 toxicity in the valspodar-treated group.45 Amonafide is a topoisomerase II inhibitor that is not affected by P-gp–mediated efflux. In combination with cytarabine, it produced a CR in a significant number of patients with secondary AML, relapsed AML, and chronic myeloid leukemia blast crisis.46 Amonafide has an excellent toxicity profile and does not have the potential cardiac toxicities of anthracyclines or anthracenediones.
Finally, a new class of agents, the parthenolides, is in preclinical testing. This drug increases oxygen stress, inhibits NF-κB, and activates p53, thus inducing leukemic stem cell apoptosis selectively over normal hematopoietic stem cells. A dimethylamino-parthenolide congener is orally bioavailable and has shown activity in murine and canine models.47
Conclusions
Dose intensification has improved the outcomes of patients who are undergoing therapy for AML. In young adults, better supportive care has enhanced the ability to deliver these higher doses of drugs in induction and consolidation. Significant progress has been made for patients under the age of 50 years and for those with favorable and intermediate-risk cytogenetics. However, these improvements have fallen short for patients over age 50 years and for those with unfavorable risk cytogenetics and/or additional molecular abnormalities. Targeted therapy with gemtuzumab ozogamicin has not impacted remission or survival results. Future use of gemtuzumab ozogamicin should be only in the context of a clinical trial, with a focus on the favorable risk cytogenetic group. Determining how to best incorporate the new prognostic markers, to guide targeted therapy in induction and consolidation, is a vital strategy for future trials. To allow use of these markers to guide therapy in the induction phase, there must be a rapid turnaround time for this molecular information, because delays would be detrimental to outcomes.48 Tailoring the approach with newer targeted agents that show promise should build on the successes of these most recent trials. Agents that are proven safe and effective in phase I/II trials should be carried forward to randomized phase III studies in the cooperative group setting.
Disclosure
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drugs: None disclosed.
Correspondence
Hugo F. Fernandez, MD, Department of Blood and Marrow Transplantation, H. Lee Moffitt Cancer Center, 12902 Magnolia Dr., FOB 3, Tampa, FL 33612; Phone: (813) 745-6012; Fax: (813) 745-5644; e-mail: hugo.fernandez@moffitt.org