Abstract
Care of the patient with massive bleeding involves more than aggressive surgery and infusion of large amounts of blood products. The proper management of massive transfusions—whether they are in trauma patients or other bleeding patients—requires coordination of the personnel in the surgical suite or the emergency department, the blood bank, and laboratory.
Role of the Massive Transfusion Protocol
The best way to coordinate all the parts of management of the massively bleeding patient is to have a well defined massive transfusion protocol (MTP). This protocol should include the following elements:
Who can initiate the protocol?
What blood products should be released?
How are laboratories to be monitored?
Details involved in deciding these elements are discussed further (Table 1). The rationale for having a protocol is that during the “heat of battle” is not the time to figure out how to rapidly assess the patient and get blood products.
Data for four studies support the logical notion that protocols are associated with better outcomes. Riskin et al1 showed that implementation of a MTP improved mortality and had a faster time to first cross-matched blood product transfused despite no change in the fresh frozen plasma:red blood cell (FFP:RBC) ratio. Use of an MTP as reported by Cotton et al2 reduced 30-day mortality and length of stay. In their series, patients received more blood products in the operating room, but less afterward. Complications such as sepsis and multiorgan system failure were also lower. Dente et al3 also showed improved survival with more blood transfused earlier to patients in their resuscitation. Finally, O'Keefe et al4 showed improved turn-around times and less blood product use with the introduction of an MTP that included use of recombinant activated factor VII.
A recent survey showed that 85% of trauma centers have MTP, but most of these have been implemented only in the last 5 years. There is considerable variation in protocols in all aspects, from who could activate the protocol to what products were released.5 The authors felt this inconsistency in the protocols was both due to the lack of national standards and disagreement about the best products to transfuse to patients. In response to the lack of published protocols, Malone et al6 propose an MTP that was based on a 1:1 FFP:RBC ratio, but admitted the lack of randomized clinical trial data to support this plan.
Crucial Personnel to Involve
At a minimum, an empowered representative from the emergency department, surgery, anesthesia, transfusion service, and laboratory medicine should be involved in development of a protocol. Although there may be disagreement on subtle issues, it is absolutely crucial that there be consensus among all parties on the basic protocol, especially issues involving blood product selection and laboratory monitoring. For example, if the MTPs call for blood product selection based on laboratory monitoring with the classics tests (ie, activated partial thromboplastin time [aPTT]), while the surgical team believes in fixed-ratio production selection augmented by thromboelastography (TEG), this will lead to conflict.
The first point of a protocol is how the protocol is initiated. One approach is that only a key point person can initiate the protocol (eg, the lead trauma surgeon). This allows for precise use of the protocol for patients the surgeon deems appropriate. This approach is efficient in that it quickly identifies the bleeding patient as someone needing increased attention and the surgeon can quickly coordinate care. The downside is that there may be a delay in activation of the protocol until the surgeon arrives, and it may be difficult to activate the protocol for other massive bleeding situations, such as obstetrical hemorrhage.
Another approach is to have a range of individuals who can perform protocol activation. This can vary from a select group, such as only surgeons or emergency room physicians, to a broad range of any residents or health care provider. In some centers, the protocol can be activated if the transfusion service notes that a large number of blood products are being sent to a specific patient.7 The approach of multiple ways to activate the protocol has the advantage of potentially faster protocol activation. Several disadvantages are that, if the lead surgeon or anesthesiologist is unaware of protocol activation, this may result in confusion and inappropriate activation will lead to wastage of product. One potential solution to this issue is to have a limited range of people—who are familiar with the protocol—who are able to activate the protocol. No matter who activates the protocol, there should be a single, defined method (most often a phone call) to the transfusion service to alert them to the need to initiate the protocol.
Crucial Transfusion Service Decisions
No matter if the underlying philosophy of product choice selection is fixed ratio-based or test result-guided,8 the most challenging demand for the Transfusion Service is supplying FFP in a timely manner. Unlike RBCs and platelets, which can be issued to the patient “off the shelf,” FFP requires 20 to 30 minutes to thaw. This can lead to the situation in which the rapidly bleeding patient may have received 10 units of packed RBCs or more before their first unit of FFP is transfused. The only way to immediately issue plasma with RBCs is to keep some thawed plasma available for release. A common choice is to always keep some units of AB FFP thawed to be part of the initial deployment of blood products. FFP can be used for up to 24 hours after being thawed. After that, it can be relabeled as “thawed plasma” and stored for four more days.9,10 Based on one small study, there appears to be no significant loss of coagulation factor activity with use of thawed plasma.11 A difficulty with this approach is that only AB plasma is the “universal donor,” and this is an infrequent blood type. Another option is liquid plasma that is plasma that is never frozen and has a 26-day outdate.12 This allows immediate transfusion of AB plasma to patients while they are being typed. For transfusion services with a high number of massive transfusions, units of the most common blood types—O and A—can be kept thawed to allow rapid use of these products as soon as the blood type is known.13 Once the MTP is activated, plasma should be rapidly thawed because units are issued by the service to allow “keeping ahead” of expected use. With a standard operating procedure in place for using thawed plasma, wastage of plasma should be minimal.
Six to 10 units of type O should be available to for rapid use. Rh+ (D+) blood should be empirically issued to all patients, except woman of childbearing age to conserve less common blood products. However, if the rate of transfusion exceeds the inventory of type Rh– (D–) blood, then Rh+ (D+) should be issued. In the trauma setting, the risk of developing anti-D antibodies is much lower than in nontrauma settings (∼ 80% vs 22%).14
Crucial Laboratory Decisions
Empiric selection and issuing of products is crucial for the initiation of the MTP. This is because it takes time to perform laboratory testing so the test results reflect not the current status of the patient, but where they were 30 or more minutes ago. Despite the fundamental role of empiric blood product choice in massive transfusions, there is still a role for laboratory management. Patients with preexisting coagulopathy (liver disease, etc) may require more aggressive resuscitation.15 As the massive transfusion proceeds, it may be prudent to ensure that platelets and fibrinogen are being adequately replaced.
A basic choice is what laboratory tests will be used during a massive transfusion to guide product selection. The “classic” tests—RBC count, platelet count, INR (international normalized ratio), aPTT, and fibrinogen—have the virtues of familiarity, years of standard protocols guiding transfusion management, and are available in most laboratories (Table 2). The downsides are that many factors can influence these labs, especially the INR and aPTT, so labs may not reflect fully all aspects of coagulation (eg, fibrinolysis); those labs are not standardized for trauma coagulopathy.16 Also, they are performed at 37°C, whereas many trauma/massive transfusion patients are hypothermic.
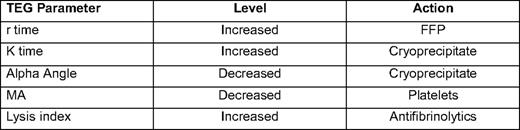
Another method of testing for coagulopathy that has received increasing attention over the past few years is TEG.17 TEG and technologies similar to it use a method to measure the increased resistance seen when whole blood clots. TEG is a unique laboratory test that examines whole blood thrombus formation and lysis.18 TEG is performed by placing a 0.35 mL of whole blood into a oscillating container with a pin that measures the force of thrombus formation. TEG measures five parameters:
r time: time from starting TEG until clot formation.
K time: time between tracing going from 2 mm to 20 mm.
Alpha angle: slope of tracing between r time and K time.
Maximal amplitude: greatest amplitude of TEG tracing.
Whole blood lysis index: amplitude of tracing 60 minutes after MA.
Most modern TEG machines automatically calculate these parameters. TEG allows rapid point-of-care testing of coagulation (all results except lysis available in 5–10 minutes) and is particularly useful in assessing fibrinolysis. The major drawbacks are that it uses fresh whole blood, so the sample must be run within minutes of being drawn. Some protocols use citrated samples, which can allow the TEG to be located in a central laboratory; but, one group has reported less reliable results with this technique.19 Also, given its selective use, data reported by the TEG may be difficult for those not trained in its use to interpret, although there are an increasing number of studies being published using TEG-based transfusions protocols that should help with this difficulty17,20 (Table 3).
Because the TEG is a point-of-care test, one decision that needs to be made is who is responsible for maintenance and quality control. In some centers laboratory medicine buys, maintains, and performs quality assurance testing of the TEG under the laboratory testing license. In others, individual services—such as anesthesia, trauma, or cardiac surgery—own and service the TEG. In this situation, it needs to be clear who ultimately is responsible for documenting quality control testing. Another decision that needs to be made is who is actually responsible for running the test? For example, if anesthesia technicians are expected to perform the assay, this will require training, documenting of competence, and the loss of their services in the operating room while they are running the test.
No matter what laboratory monitoring is chosen, there needs to be an effective way to communicate the lab findings to those in charge of running the MTP and to record the results. A simple, but effective, method is to keep a running flow chart of laboratories in a highly visible location. Some centers have even chosen to use a large whiteboard in the operating room to record laboratory values so all can see and react to the results.
Crucial Logistical Decisions
A very basic issue is getting blood from the transfusion service to the patient, and getting blood samples from the patient to the laboratory. All trauma protocols should require a blood sample to be sent for basic coagulation laboratories and sent for blood bank for typing. In the emergency departments of trauma units, there can be premade “admission packets” that contain a patient medical record number to be assigned to the patient.13 For immediate use, it is helpful to depot blood products away from the blood bank and near the trauma operating room. Some facilities have a Pyxis-like blood refrigerator where cross-matched and emergency uncross-matched units can be stored. Specific units can only be obtained by use of a barcode to help prevent transfusion errors. An additional approach is the “box of blood” or “transfusion package” approach.7 This has standard bundles of blood products issued as the massive transfusion proceeds. For example, a first transportation container could contain 6 to 10 uncross-matched type O+ RBCs and 6 units of thawed plasma; the second container would have these products plus a unit of platelets (obviously kept outside the cooler). As soon as the blood type is known, then type-specific units are released. There have been several variations on this theme published. For example, Stanford dispenses RBC:FFP: pheresis platelets in a 6:4:1 ratio at every request for blood.12
For any massive transfusion, there needs to be a dedicated person in the operating suite to draw the blood samples, label them properly, and send them off for testing. The laboratory needs to be prepared to immediately run the samples and call in the result to the person running the transfusion while the transfusion service sets up and issues the products.
A not-so-obvious problem, but one that can cause major issues, is simply getting samples and blood back and forth from the patient to the laboratory service. In larger institutions, these can be separated by a considerable distance. Pneumatic tube systems can handle test samples and most can carry blood products, but can have reliability issues. Therefore, in an emergency situation, a transportation person should be responsible for delivery of the blood product and laboratory samples. An important item in any MTP is identification of people who will transport laboratory and blood products. One method is to identify a person from the each of the hospital units where a massive transfusion is most likely to occur—emergency department, surgery, trauma unit—to be in charge of “running” products. This is another area where preplanning will lessen confusion during the massive transfusion.
Crucial Parameters to Monitor
Finally, there needs to be a decision on what is to be monitored with a protocol to make sure it is working. Time from initiation to first cross-matched product is a common and important goal. Increasingly, plasma:RBC and platelet:RBC ratios are being followed. “Wasted units” are another crucial parameter to track. There should be regular meetings of all the affected parties to ensure monitoring and make needed adjustments to adverse trends or new information. Recently, Cotton et al21 reported on their performance improvement process by monitoring the following seven processes:
Activation by attending trauma surgeon.
Type and screen sent from emergency department.
RBC:FFP ratio = 3:2.
RBC: pheresis platelets = 1:5.
Blood products received in a timely fashion.
Unused products stored appropriately.
Timely deactivation of protocol.
Their process improvement system allows identification of what processes were working and where improvement efforts needed to be targeted.
Putting it All Together
An effective MTP is a team effort between the emergency department, surgery/trauma unit, and the laboratory. Time spent in creating, maintaining, and updating an MTP will provide benefits to all concern, especially the massively bleeding patient.
Disclosures
Conflict-of-interest disclosure: Author has received honoraria, and has consulted Sanofi-Aventis, Amgen, GlaxoSmithKline, and Alexion Pharmaceuticals.
Off-label drug use: None disclosed.
Correspondence
Thomas DeLoughery, MD, FACP, Professor of Medicine, Pathology, and Pediatrics, Divisions of Hematology/Oncology and Laboratory Medicine, Hematology L586, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201-3098; Phone: (503) 494-8534; Fax: (503) 494-3257; e-mail: delought@ohsu.edu