Abstract
Renal impairment is a common complication of multiple myeloma. Chronic renal failure is classified according to glomerular filtration rate as estimated by the MDRD (modification of diet in renal disease) formula, while RIFLE (risk, injury, failure, loss and end-stage renal disease) and AKIN (acute renal injury network) criteria may be used for the definition of the severity of acute renal injury. Novel criteria based on estimated glomerular filtration rate measurements are proposed for the definition of the reversibility of renal impairment. Renal complete response (CRrenal) is defined as sustained (i.e., lasting at least 2 months) improvement of creatinine clearance (CRCL) from under 50 mL/min at baseline to 60 mL/min or above. Renal partial response (PRrenal) is defined as sustained improvement of CRCL from under 15 mL/min at baseline to 30 to 59 mL/min. Renal minor response (MRrenal) is defined as sustained improvement of the baseline CRCL of under 15 mL/min to 15 to 29 mL/min or, if baseline CRCL was 15 to 29 mL/min, improvement to 30 to 59 mL/min. Bortezomib with high-dose dexamethasone is considered the treatment of choice for myeloma patients with renal impairment and improves renal function in most patients. Although there is limited experience with thalidomide, this agent can be administered at the standard dosage to patients with renal failure. Lenalidomide, when administered at reduced doses according to renal function, is effective and can reverse renal impairment in a subset of myeloma patients.
Definitions
Multiple myeloma (MM) is characterized by the malignant proliferation of plasma cells that usually produce a monoclonal immunoglobulin. Renal impairment is a common feature of MM.1 In the era of conventional chemotherapy, several studies have shown that renal impairment is associated with inferior survival.2–5 The introduction of novel agents has led to an improved survival of patients with MM,6,7 and there is preliminary evidence that this improvement also occurs in patients with renal impairment. Some studies have indicated that reversibility of renal impairment is associated with improved survival.8–10 Depending on the definition of renal insufficiency, this complication is reported in 15% to 40% of MM patients. At diagnosis, 30% to 40% of patients with symptomatic MM have a serum creatinine above the upper limit of normal, and approximately 20% have a serum creatinine above 2 mg/dL. Less than 10% of patients present with severe renal failure.11 Renal impairment can also develop over time, and an estimated 25% to 50% of patients are affected during the course of their disease.12
Serum creatinine is easily measured, and in several trials it has been used to define renal impairment in MM. A serum creatinine above 2.0 mg/dL represents one of the “CRAB”—calcium, renal (creatinine), anemia (hemoglobin), and bone lesions—diagnostic criteria for symptomatic MM requiring therapy.13 However, serum creatinine is not directly related to renal function and depends on factors such as age, gender, and muscle mass.12 The glomerular filtration rate (GFR) is a more accurate assessment parameter, providing a true reflection of renal function. GFR in healthy subjects is considered to be in the range of 90 to 130 mL/min/1.73 m2. The standard method used to measure GFR is unsuitable for clinical practice due to the need for continuous infusion, multiple blood samples, and expense.14 Creatinine clearance (CRCL) is defined as the volume of plasma that is completely cleared of creatinine in a unit of time (usually milliliters per minute). CRCL may overestimate GFR due to the additional tubular secretion of creatinine, a mechanism that becomes relatively more important when renal function declines.14,15
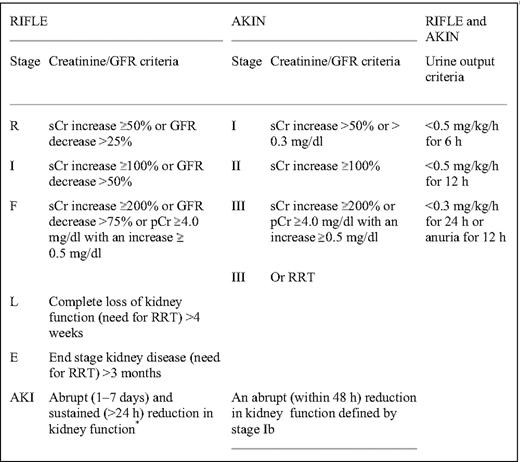
CRCL has been largely replaced by prediction formulas of GFR such as the C-G (Cockcroft-Gault) and MDRD (modification of diet in renal disease) study equations (Table 1).14 Because the C-G and MDRD equations have limitations, especially in the normal or near-normal GFR range and in kidney transplant recipients, other prediction equations based on serum cystatin-C values may be more sensitive GFR surrogate markers.15 Almost 65% of MM patients at diagnosis have high cystatin-C serum levels.16 Estimating GFR based on both serum cystatin-C and serum creatinine seems to be more accurate than estimations based only on serum creatinine.15 Both the Kidney Disease Improving Global Outcomes (KDIGO) and the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (K/DOQI) recommend that chronic kidney disease (CKD) should be classified in stages based on GFR (Table 2),17 and MDRD is the recommended formula for CRCL evaluation. However, both the MDRD equation and the five stages of the KDIGO classification have been validated only in patients with CKD, not in those with acute renal injury (AKI). Several patients with MM cast nephropathy present with AKI and rapid deterioration of GFR within a few days. For these patients, RIFLE (risk, injury, failure, loss, and end-stage renal disease) and AKI network (AKIN) criteria are likely to be more appropriate to define the severity of AKI in MM18 (Table 3). However, these criteria have not been validated in myeloma patients.
Prediction equations for GFR currently used or proposed for use in clinical practice
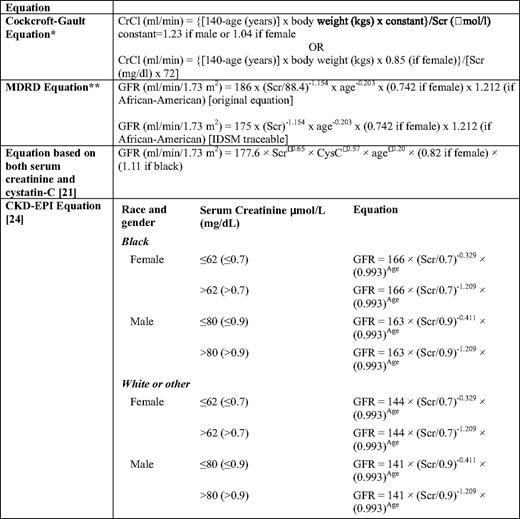
CRCL, creatinine clearance; Cys-C, serum cystatin-C; GFR, glomerular filtration rate; CRCLScr, serum creatinine
*The Cockcroft formula can also be expressed per 1.73 m2 body surface area.
†The original MDRD equations were validated with the modified kinetic Jaffe method used to assess creatinine. Because this commonly used assay overestimates creatinine, a different prediction equation has to be used when the (more expensive) enzymatic assay traceable to the reference method GC-IDMS is used to assess creatinine. GFR can be estimated easily using MDRD available online; for example, see http://www.mdrd.com
Classification of chronic renal disorders*
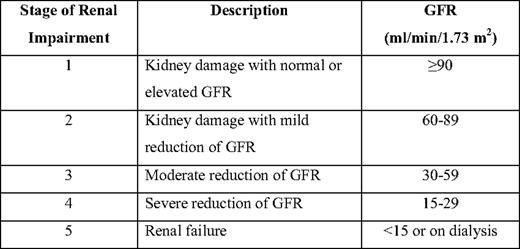
*Stage 5 is also defined as end-stage renal disease (ESRD), while stage 4 is defined as pre-ESRD.
Pathophysiology of Renal Impairment in Myeloma
Renal dysfunction in MM results primarily from the toxic effects of monoclonal light chains on the kidney, in addition to other contributing factors such as dehydration, hypercalcemia, hyperuricemia, the use of nephrotoxic drugs (nonsteroidal anti-inflammatory drugs, antibiotics, contrast media, etc.), and, rarely, myeloma cell infiltration or hyperviscosity.19 Cast nephropathy is the main cause of renal impairment in MM (∼90% of cases) and is characterized by tubular atrophy and tubular-interstitial fibrosis. Normally, light chains are filtered by the glomerulus, reabsorbed, and catabolized by the cells of the proximal tubule. In myeloma, the abundance of light chains overwhelms the capability of the proximal tubular cells, raising the concentration of light chains delivered to the distal tubule. The characteristic finding of “myeloma kidney” is the presence of myeloma casts, composed mainly of light chains and Tamm-Horsfall protein in the distal tubules and collecting ducts.19 Several studies have revealed the role of proximal tubule cells in the pathogenesis of cast nephropathy. In proximal tubule cells, prolonged exposure to myeloma light chains induces apoptosis, DNA degradation, and the production of the inflammatory and proinflammatory cytokines that initiate renal interstitial fibrosis and tubular destruction.20 Some myeloma patients present with acute oliguric renal failure that is often associated with significant dehydration and with massive deposition in distal and (mainly) proximal tubules. Acute cast nephropathy is generally associated with poor outcome.
Light-chain glomerulopathy is caused by the deposition of immunoglobulins either in the form of amyloid or non-amyloid. In both glomerulopathies, the development of nonselective proteinuria is the dominant syndrome. Amyloid deposits predominate within the glomeruli and give a positive Congo red staining. In monoclonal immunoglobulin deposition disease (MIDD), the glomerular deposits of immunoglobulin light or heavy chains are non-fibrillar and Congo red negative. In MIDD, granular depositions of light chains are observed within the mesangial areas, whereas a thickening of the peripheral basement membrane may resemble type II membranoproliferative glomerulonephritis or diabetic lesions. Diagnosis requires renal biopsy with ultrastructural studies. In contrast to amyloidosis, in which the light chain is of the lambda type in 80% of cases, in MIDD the light chain is usually of the kappa type.19
At this point it is crucial to mention that the pathogenesis of renal impairment is multifactorial, at least in a subset of patients with myeloma. Thus, the treating physician has to address the underlying cause of renal dysfunction in these patients. This is important, as we cannot expect an improvement of non-myeloma-related renal impairment by anti-myeloma therapy.
Diagnostic Evaluation of Patients with Renal Impairment
Serum creatinine, urea, sodium, and potassium should be measured and GFR should be estimated based on the MDRD formula (Table 1) in all myeloma patients at diagnosis and at regular follow-up visits. Electrophoresis and immunofixation of a sample from a 24-h urine collection are also necessary. If the patient has proteinuria that consists mainly of light chains, a renal biopsy is not necessary. The presence of nonselective proteinuria or significant albuminuria may support the suspicion of amyloidosis or MIDD; therefore, in patients with nephrotic syndrome with or without renal failure, a biopsy of the subcutaneous fat or a rectal biopsy is needed for the confirmation of amyloidosis. If amyloidosis is not demonstrated, a kidney biopsy is needed to search for amyloid, MIDD, or an unrelated glomerulopathy such as glomerulonephritis or diabetes nephritis.
Prognosis and Reversibility of Renal Failure
Until recently, the median survival time of MM patients with renal insufficiency was less than 1 year,3–5,8,11 and patients requiring dialysis had a particularly poor prognosis.9 However, the prognosis for these patients has improved recently due to the availability of more effective treatments for MM and to improvements in supportive care. Prognosis, however, remains tied to the reversibility of renal impairment, although this has not been confirmed in all studies.
When the reversal of renal impairment is defined as a decrease in the serum creatinine level to under 1.5 mg/dL, the frequency of reversal of renal failure ranges from 20% in the past to 73% in the most recent series.8,10 These results were obtained before the novel CKD classification of chronic renal impairment. With this new classification, novel criteria for the improvement of renal function have been proposed.21 CRrenal is defined as sustained (i.e., lasting at least 2 months) improvement of CRCL from under 50 mL/min at baseline to 60 mL/min or above. PRrenal is defined as sustained improvement of CRCL from under 15 mL/min at baseline to 30 to 59 mL/min. MRrenal is defined as sustained improvement of baseline CRCL of under 15 mL/min to 15 to 29 mL/min or, if baseline CRCL was 15 to 29 mL/min, improvement to 30 to 59 mL/min. In amyloidosis and MIDD, the time to reversal is usually much longer than in patients with myeloma cast nephropathy.
Management of Myeloma Patients with Renal Impairment
Supportive Care
Adequate hydration, urine alkalinization, and management of hypercalcemia are important supportive measures for the management of myeloma patients with acute renal failure, and may restore renal function in some patients.19,22
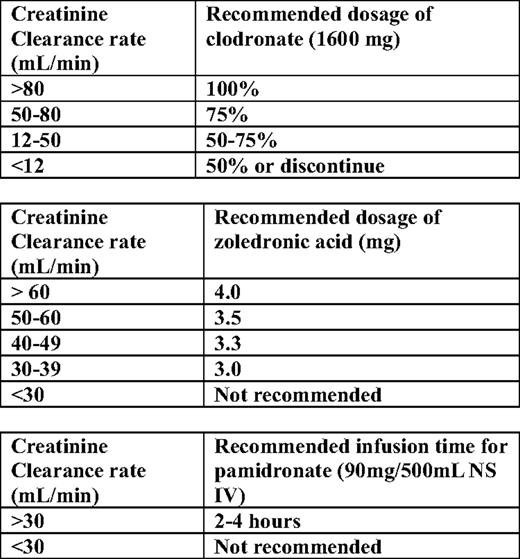
For the treatment of hypercalcemia in myeloma patients with renal impairment, adequate hydration is necessary. Bisphosphonates have to be given according to guidelines for estimated GFR (eGFR); intravenous bisphosphates should be retained in patients with GFR under 30 mL/min. For zoledronic acid, recommendations for dose reduction have been issued for patients with GFR between 30 and 60 mL/min (Table 4).22 Calcitonin has also to be considered in the management of these patients; if needed, loop diuretics can be administered if the patient has been adequately hydrated and the fluid balance is monitored carefully to prevent dehydration.
Drugs that may contribute to renal damage, such as nonsteroid anti-inflammatory drugs, IV contrast dyes, aminoglycosides, or other antibiotics that have significant renal excretion, should be avoided. If a myeloma patient with renal failure needs intravenous contrast media for diagnostic purposes, dialysis immediately after the procedure is recommended.22
Mechanical Approaches
Mechanical means of treating MM patients with renal impairment include long-term dialysis, plasmapheresis, and novel dialysis filters for the removal of free light chains. Long-term dialysis is a worthwhile supportive measure for patients with MM and end-stage renal disease. If we exclude patients with dialysis-dependent renal insufficiency who die within the first 2 months of therapy (approximately 30% of the total), then long-term dialysis in combination with conventional anti-myeloma therapy can lead to a median survival time of approximately 2 years.19,22 The role of plasmapheresis has been evaluated in the context of prospective clinical trials, and no clear benefit was detected.23 The removal of free light chains with dialysis is another approach: a new hemodialysis membrane that seems to remove circulating light chains efficiently has been recently tested in the MM setting with encouraging results.24
Systemic Therapy
Conventional and High-Dose Therapy.
The response rate to alkylator-based conventional chemotherapy is lower in MM patients with renal insufficiency than in those with normal renal function (40% vs. 60%).8 The combination of vincristine + doxorubicin + dexamethasone (VAD), or cyclophosphamide + dexamethasone, or even dexamethasone alone have rapid anti-myeloma activity and low renal excretion. In a series of 41 MM patients with renal impairment treated with high-dose dexamethasone-containing regimens, renal failure was reversible in 73%.10 High-dose therapy with autologous stem cell transplantation is another option for eligible MM patients with renal impairment, with a 5-year overall survival (OS) of 35%.25
Bortezomib-based Regimens.
Bortezomib can be administered at the full approved dose and schedule in patients with impaired renal function. Studies indicate that bortezomib is effective and safe in patients with renal impairment and that it can improve renal function. In a subanalysis of the SUMMIT (Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy) and the CREST (Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma) phase 2 studies, 3/10 (30%) patients with CRCL of 30 mL/min and under demonstrated responses to treatment, compared with a 45% overall response rate in patients with baseline CRCL of above 80 mL/min. Discontinuation rates and adverse-event profiles were similar between patients with CRCL above 80 mL/min and those with CRCL 50 mL/min and under.26 In a report of the phase 3 APEX (Assessment of Proteasome Inhibition for Extending Remissions) study of bortezomib compared with high-dose dexamethasone, time to progression (TTP), OS, and safety were comparable between patients with CRCL of 50 mL/min or below and those with CRCL above 50 mL/min, although there was a trend toward shorter TTP and OS in patients with CRCL of 50 mL/min and below. Response rates with bortezomib were similar and the time to response very rapid (0.7–1.6 months) in all subgroups. Furthermore, bortezomib showed greater efficacy compared with high-dose dexamethasone in all subgroups. Toxicity following bortezomib treatment was similar in all subgroups, but the incidence of adverse events and discontinuations was higher in patients with moderate to severe renal impairment compared with those with mild or no renal impairment.27 In a retrospective series of 24 patients with relapsed/refractory MM and dialysis-dependent renal failure who were treated with bortezomib alone or bortezomib-based regimens, Chanan-Kahn et al. reported an overall response rate of 75%, with 30% CRrenal or near CRrenal. Three patients became dialysis independent following bortezomib therapy. Importantly, the toxicity profile was similar to that reported in patients with normal renal function treated with bortezomib.28
Ludwig et al. reported the reversal of light-chain-induced acute renal failure with bortezomib-based therapy in 5/8 MM patients.29 During the ASH 2009 meeting, the same group reported the effect of a BDD (bortezomib + doxorubicin + dexamethasone) regimen on the reversal of light-chain-induced acute renal failure. They found that 42/58 (72%) patients achieved a renal response (36% CRrenal, 9% PRrenal, and 27% MRrenal), and 3/9 dialysis-dependent patients became dialysis independent. Renal response was observed at a median time of 38 d, while the median time to CRrenal was 111 d.30 Dimopoulos et al. analyzed 46 consecutive MM patients who presented with renal impairment and received bortezomib with dexamethasone with or without other agents. Renal response was documented in 59% of patients (30% achieved CRrenal) within a median of 11 d, while 2/9 patients who required dialysis became dialysis independent. Toxicities were similar to those seen in MM patients without renal failure who received bortezomib-based regimens.21 In a retrospective study, Blade et al. reported that the response rate and median TTP were comparable in patients with renal insufficiency (n = 193) treated with bortezomib plus pegylated liposomal doxorubicin compared with patients treated with bortezomib alone. There was an improvement of renal function for patients with renal insufficiency in both treatment arms; however, patients with impaired renal function were at an increased risk of a drug-related serious adverse event (28% vs. 19% for CRCL < 60 and ≥ 60 mL/min, respectively).31
In another recent retrospective analysis, bortezomib-based regimens were given in 117 MM patients with renal impairment, including 14 patients who required dialysis. The rate of bortezomib discontinuation in cases with severe, moderate, and mild renal impairment was 11%, 5%, and 0%, respectively. At least a PRrenal was documented in 83/113 evaluable patients (73%), including 27% of the CRrenal or near CRrenal patients. The overall response rate was similar across different subgroups of renal function. Reversal of renal impairment was documented in 41% of patients after a median of 2.3 months, and the 2-year OS was 51%.32 Dimopoulos et al. reported the results of the subgroup analysis of the VISTA (Velcade as Initial Standard Therapy in multiple myeloma: Assessment with melphalan and prednisone) phase 3 trial on the effect of the VMP (bortezomib + melphalan + prednisone) and the MP (melphalan + prednisone) combination on renal impairment. At baseline, 6%/4% and 27%/30% of patients had an eGFR of 30 mL/min or under and 31 to 50 mL/min in VMP/MP, respectively. Response rates were higher and TTP and OS were longer with VMP compared with MP across renal cohorts. Response rates with VMP and TTP in both arms did not appear significantly different between patients with GFR of 50 mL/min and under and those with GFR above 50 mL/min, while OS appeared somewhat longer in patients with normal renal function in both arms of the study. Renal impairment reversal, defined as a baseline eGFR under 50 mL/min improving to above 60 mL/min, was seen in 49/111 (44%) patients receiving VMP versus 40/116 (34%) of those receiving MP. Younger age (<75 years) and eGFR 30 mL/min or above were independently associated with higher reversal rates. In both arms, rates of grade 4 and 5 adverse events appeared higher in patients with renal impairment; with VMP, rates of discontinuation or bortezomib dose reduction due to AEs did not appear to be affected.33 Bortezomib has also been shown to significantly reduce levels of cystatin-C, a marker of GFR and therefore an early marker for renal impairment, in patients with relapsed MM.16
IMiD-based Regimens.
Thalidomide is the first immunomodulatory drug (IMiD) with proven activity in MM. Thalidomide pharmacokinetics are not affected by renal impairment, and thus no dose reduction is required in MM patients with renal impairment. There are limited data on the efficacy of thalidomide-based regimens in MM patients with renal impairment. In a single-center case series (N = 20) of patients with relapsed/refractory MM and renal impairment (defined as serum creatinine above 2 mg/dL), treatment with thalidomide alone (n = 8) or thalidomide plus dexamethasone (n = 12) resulted in 45% PRrenal and 30% MRrenal. The median duration of response was 7 months, while 12/15 responding patients had improved renal function defined as serum creatinine under 2 mg/dL; two additional patients on chronic hemodialysis showed a reduction of serum creatinine. Toxicities were similar to those reported with thalidomide in patients with normal renal function.34 Kastritis et al. also observed a reversal of renal failure with thalidomide in combination with high-dose dexamethasone with or without bortezomib in 80% of previously untreated patients.10 Treatment with thalidomide has been associated with severe hyperkalemia in some patients with renal impairment, particularly those undergoing dialysis.35,36
Lenalidomide is another effective agent for the management of MM. It is mainly excreted by the kidneys, through both glomerular filtration and active tubular secretion. The following dose adjustments have been recommended for MM patients with renal insufficiency: reduce the dose to 10 mg/d in patients with CRCL between 30 and 50 mL/min; to 15 mg every other day in patients with CRCL under 30 mL/min not on dialysis; and to 5 mg once daily in patients requiring renal dialysis.19 Information on the effect of lenalidomide-based regimens on myeloma patients with renal impairment is limited, because in the majority of phase 2/3 studies serum creatinine above 2 mg/dL was an exclusion criterion. In a combined analysis of the MM-009 and MM-010 phase 3 studies of lenalidomide and dexamethasone versus dexamethasone alone in patients with relapsed MM, efficacy and safety were assessed in patients with normal renal function and with mild, moderate, and severe renal impairment (assessed as CRCL > 80, 50–79, 30–49, and <30 mL/min, respectively). The renal subgroups did not significantly differ regarding overall response rate (50%–63%) or response quality (≥ vgPR 30%–38%). In all renal subgroups except patients with severe renal impairment, TTP, progression-free survival, and OS did not differ significantly from patients with normal renal function. Patients with severe renal impairment experienced an increased incidence of thrombocytopenia, required more frequent lenalidomide dose reductions or interruptions, and had shorter OS than those with no renal impairment (p < 0.01). Importantly, improvement in CRCL by at least one level was experienced by 119/174 (68%) patients with mild to severe renal impairment.37 Similarly, in a series of patients with relapsed/refractory MM who received lenalidomide plus dexamethasone, 3/12 (25%) patients with renal impairment (CRCL < 50 mL/min) achieved CRrenal and 2/12 (16%) achieved MRrenal.38 In a Spanish retrospective analysis of 15 dialysis-dependent MM patients who received lenalidomide plus dexamethasone, 57% achieved a response, and one patient became independent of dialysis.39 In a phase 2 study of newly diagnosed MM patients treated with lenalidomide plus dexamethasone, baseline CRCL of 40 mL/min and under was associated with grade 3 or higher myelosuppression and an 8.4-fold increased likelihood of lenalidomide dose reduction compared with patients with a CRCL above 40 mL/min.40
Conclusions
Renal impairment is a common and severe complication of MM. Novel criteria based on eGFR measurements are recommended for the definition of the reversibility of renal impairment. High-dose dexamethasone therapies are highly active in myeloma patients with renal impairment. Available data support the safety and efficacy of bortezomib-based therapies in this setting, so bortezomib + dexamethasone is the recommended treatment for myeloma patients with renal impairment of any grade. Lenalidomide is a feasible and effective treatment option for patients with mild to moderate renal impairment, but it should be administered at the recommended reduced dose based on renal function. Thalidomide is also an option for patients with severe renal impairment, although the data on this are less extensive. Bortezomib and IMiD combinations with high-dose dexamethasone have also shown superior anti-myeloma activity to VAD, although no comparative studies have been performed specifically in patients with renal impairment. High-dose therapy with autologous stem cell transplantation can be an option for such patients; the high-dose regimen should consist of melphalan 140 mg/m2 and the procedure should be restricted to patients younger than 60 years of age with chemosensitive disease and good performance status.
Disclosures
Conflict-of-interest disclosure: MAD has received honoraria and has served on advisory committees of Millenium, Ortho Biotech, and Celgene. ET has received honoraria from Janssen-Cilag and Celgene. Off-label drug use: bortezomib and lenalidomide as frontline treatment in patients with MM.
Correspondence
Meletios A. Dimopoulos, MD, Department of Clinical Therapeutics, University of Athens School of Medicine, 80 Vas. Sofias Avenue, Athens, 11528, Greece; Phone: 30–210-3381540; Fax: 30–210-3381511; e-mail: mdimop@med.uoa.gr