Abstract
The recent recognition of genetic defects in telomeres and telomere repair in multiple human diseases has practical implications for hematologists and oncologists and their patients; consequences for future clinical research in hematology and other subspecialties; and even importance in the interpretation of animal experiments involving cell propagation. Telomere diseases include constitutional marrow failure as dyskeratosis congenita, some apparently acquired aplastic anemia, myelodysplasia and acute myeloid leukemia; pulmonary fibrosis; and hepatic nodular regenerative hyperplasia and cirrhosis. Accelerated telomere attrition is a likely pathophysiology of cancer arising from chronic inflammation. Telomerase can be modulated by sex hormones, which may explain the activity of androgens in marrow failure. Measurement of telomere length of peripheral blood leukocytes is a simple screening clinical assay. Detection of a mutation in a patient has implications for therapy, prognosis, monitoring, and genetic counseling. For research in hematology and oncology, telomere biology could be assessed as a risk for secondary malignancies and in graft-versus-host disease, for progression in a variety of blood cancers, and as potentially modifiable by hormone replacement strategies.
History
Telomeres are essential components of linear chromosomes, but their existence was postulated years before the elucidation of the double helix. In the late 1930s, Hermann Muller and Barbara McClintock independently noted that the ends of chromosomes (termed “telomeres” or “natural ends”) lacked the “stickiness” of broken chromosomal ends. Decades later, after the discovery of the structure of DNA, Alexey Olovnikov implicated telomeres in the “Hayflick limit” of cellular duplication; further, he realized that in DNA semi-conservative replication, the replica would always be shorter than the template, eventually producing extremely short chromosomes and senescent cells; his ‘theory of marginotomy’ hypothesized that telomeres would buffer genetic loss with successive mitotic divisions and that telomere shortening could explain many aging-related disorders.” James Watson similarly concluded from observations of T7 DNA phage replication that “there was no simple way for 3′-5′ growth to reach the 3′ end of its template.” In the early 1980s, Elizabeth Blackburn and her associates solved the structure of telomeres and discovered an enzyme, telomerase, capable of countering telomere erosion by DNA synthesis through reverse transcription.
The reader is referred to our recent, more comprehensive and illustrated review of telomere biology and telomere diseases.1
A Primer of Telomere Biology
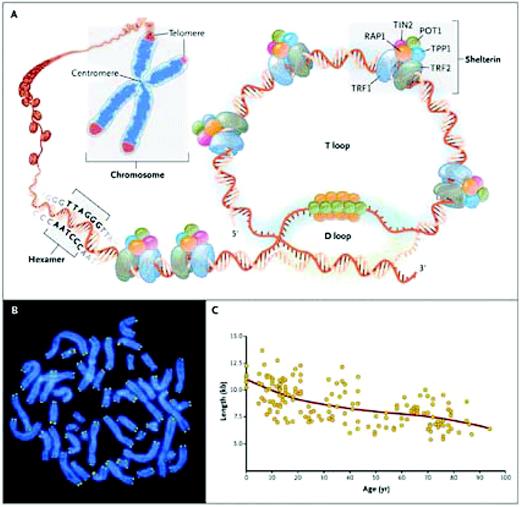
Telomeres are at the ends of linear chromosomes and composed of many (500 to 2000 in human cells) tandem repeats of a hexanucleotide—TTAGGG—in the leading 5′-strand of DNA and associated proteins, collectively termed the shelterin complex (Figure 1). The single-stranded 3′ overhang folds back to anneal with the C-rich strand of the double helix, forming the T loop. The associated shelterin proteins bind to the telomere, providing molecular signals that prevent the DNA repair machinery from mistaking the chromosome end for a double-stranded break.
Telomere structure. (A) Telomeres are located at the ends of linear chromosomes; they are composed of hundreds to thousands of tandem DNA repeat sequences: hexameric TTAGGG in the leading strand and CCCTAA in the lagging strand in humans. Protective proteins associated with telomere DNA are collectively termed shelterin (TRF1, TRF2, TIN2, POT1, TPP1, and RAP1). The 3′ end of the telomeric leading strand terminates as a single-stranded overhang, which folds back and invades the double-stranded telomeric helix, forming the T loop. (B) Telomeres can be directly visualized under the microscope at the ends of metaphase chromosomes (four telomere signals per chromosome) by fluorescence in situ hybridization (FISH). (Image provided by Peter Lansdorp, MD, PhD.) Average telomere length can be measured by several methods: a technique that combines flow cytometry and FISH (flow-FISH), Southern blotting, and a quantitative polymerase-chain-reaction (qPCR) assay. Flow-FISH can measure the telomere length in different cell subgroups, such as granulocytes or CD4+ T lymphocytes; Southern blotting reveals length and length heterogeneity; and qPCR is a rapid assay that requires very small amounts of DNA. (C) The average length of telomeres in human leukocytes varies, ranging from approximately 11 kb at birth (in umbilical-cord blood) to 6 kb at 90 years of age. Telomere loss is most rapid early in life, and over a life span it is not linear but follows a third-order polynomial. Data are from Yamaguchi et al.14 Reprinted with permission from Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365.
Telomere structure. (A) Telomeres are located at the ends of linear chromosomes; they are composed of hundreds to thousands of tandem DNA repeat sequences: hexameric TTAGGG in the leading strand and CCCTAA in the lagging strand in humans. Protective proteins associated with telomere DNA are collectively termed shelterin (TRF1, TRF2, TIN2, POT1, TPP1, and RAP1). The 3′ end of the telomeric leading strand terminates as a single-stranded overhang, which folds back and invades the double-stranded telomeric helix, forming the T loop. (B) Telomeres can be directly visualized under the microscope at the ends of metaphase chromosomes (four telomere signals per chromosome) by fluorescence in situ hybridization (FISH). (Image provided by Peter Lansdorp, MD, PhD.) Average telomere length can be measured by several methods: a technique that combines flow cytometry and FISH (flow-FISH), Southern blotting, and a quantitative polymerase-chain-reaction (qPCR) assay. Flow-FISH can measure the telomere length in different cell subgroups, such as granulocytes or CD4+ T lymphocytes; Southern blotting reveals length and length heterogeneity; and qPCR is a rapid assay that requires very small amounts of DNA. (C) The average length of telomeres in human leukocytes varies, ranging from approximately 11 kb at birth (in umbilical-cord blood) to 6 kb at 90 years of age. Telomere loss is most rapid early in life, and over a life span it is not linear but follows a third-order polynomial. Data are from Yamaguchi et al.14 Reprinted with permission from Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365.
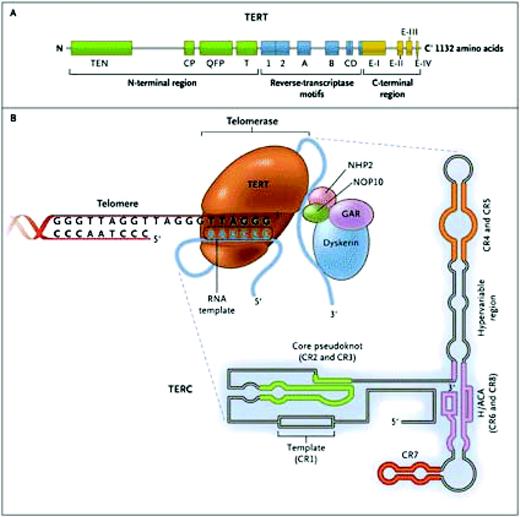
The telomerase ribonucleoprotein complex actively maintains telomere length, slowing (but not entirely preventing) telomere attrition. Telomerase consists of two copies of telomerase reverse transcriptase (TERT) and two copies of its integral RNA template (TERC), and other proteins that stabilize the complex (Figure 2). TERT copies a short region of TERC into telomeric DNA to extend the 3′ end of the chromosome. Telomerase is active in cells with high replicative demands, such as hematopoietic cells and lymphocytes. Telomere repair is highly regulated, and TERT transcription, for example, can be upregulated by sex hormones2 and transcriptional factors like c-Myc.3
The telomerase complex and its components. The enzyme telomerase reverse transcriptase (TERT), its RNA component (TERC), the protein dyskerin, and other associated proteins (NHP2, NOP10, and GAR1) are shown. Telomerase catalytically adds TTAGGG hexameric nucleotide repeats to the 3′-hydroxyl end of the telomeric leading strand, using a specific sequence in the RNA component as the template. TERT contains three major domains: the N-terminal region, the reverse-transcriptase motifs, and the C-terminal region, all containing evolutionarily conserved motifs. TERC contains 451 nucleotides in seven conserved regions (CR1 through CR7), including the template (CR1), and an H/ACA box, a hairpin nucleotide sequence characteristic of a class of small nucleolar RNAs involved in RNA processing. Reprinted with permission from Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365.
The telomerase complex and its components. The enzyme telomerase reverse transcriptase (TERT), its RNA component (TERC), the protein dyskerin, and other associated proteins (NHP2, NOP10, and GAR1) are shown. Telomerase catalytically adds TTAGGG hexameric nucleotide repeats to the 3′-hydroxyl end of the telomeric leading strand, using a specific sequence in the RNA component as the template. TERT contains three major domains: the N-terminal region, the reverse-transcriptase motifs, and the C-terminal region, all containing evolutionarily conserved motifs. TERC contains 451 nucleotides in seven conserved regions (CR1 through CR7), including the template (CR1), and an H/ACA box, a hairpin nucleotide sequence characteristic of a class of small nucleolar RNAs involved in RNA processing. Reprinted with permission from Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365.
Telomere length in cells provides a mitotic clock, a measure of the cell's replicative history; 50 to 100 base pairs are lost with each human cell division.4 Telomeres of leukocytes and some other tissues also shorten with aging of the organism, but there is considerable overlap of telomere length of the very young and the very old; telomere attrition accompanies aging, but an etiologic relationship is not established. Telomere length of leukocytes has been correlated with telomere attrition in only a few other tissues. With critical telomere shortening, even of a single chromosome, the cell no longer proliferates and becomes either senescent or undergoes apoptosis. If the cell continues to proliferate in the presence of very short telomeres, the result is genomic—really chromosomal—instability, with end-to-end chromosome fusions, translocations, and aneuploidy in vitro and malignant tumors in telomerase-deficient mice.
Quantitative assays are available for telomere length and telomerase enzymatic activity. Telomeres can be visualized by fluorescent in situ hybridization (FISH) of individual cells and in-flow cytometry of specific cell populations. Telomeres can be measured by Southern DNA hybridization and in quantitative polymerase chain reaction amplification assays. Age-matched controls are critical due to physiologic shortening of telomeres with aging. Telomerase can be measured in cells by the ability of an extract to add hexanucleotides to a complementary substrate, and enzyme activity of mutated gene products can be assessed by transfection into a cell line that lacks TERT and TERC.
Human Telomere Diseases
Aplastic Anemia.
Short-for-age leukocyte telomeres were observed in several human diseases, including in aplastic anemia, and initially explained as the consequence of “regenerative stress” or reflective of damaging chronic exposure to free oxygen radicals.5,6 A mechanism was provided by the discovery in pedigrees of children with a constitutional marrow failure syndrome, dyskeratosis congenita, of mutations in DKC1, which encodes a protein of the telomerase complex, followed by measurement of very short telomeres in these patients.7,8 The RNA template gene TERC subsequently was found to be mutated in some autosomal inheritance pedigrees,9 and in other families mutations were discovered in additional genes of the complex, NOP1010 and NHP2,11 and in a gene encoding a shelterin protein, TINF2.12 DKC1 mutations usually lead to disease presentation early in life, characteristic mucocutaneous signs, and, in the very severe Hoyeraal-Hreidarsson syndrome, neurologic abnormalities.13 In contrast, TERT mutations were first described in adult patients with apparently acquired aplastic anemia, who typically lack stereotypical physical findings or a striking family history.14 In our experience, about 10% of patients with “idiopathic” aplastic anemia have TERT or TERC mutations.
All mutations lead to very short telomeres, so that telomere length can be used as a screening test. Mutation of the X-linked DKC1 gene results in telomerase deficiency due to hemizygosity. TERT and TERC mutations act dominantly, so that heterozygosity leads to a dysfunctional telomere repair complex by a mechanism of haploinsufficiency. NHP2 and NOP10 mutations are recessive. Telomere shortening is severe with TINF2 mutations, despite normal telomerase activity. Some dyskeratosis congenita families show anticipation, early manifestation, and more severe symptoms and signs in progressive generations. Short telomeres can be inherited from a parent but, in the presence of normal telomerase, are without apparent clinical effects.15 For most telomerase gene mutations, penetrance is variable; family members with mutations range from frank disease to subtle laboratory or clinical manifestations. In some pedigrees, blood counts are normal well into middle life despite hypocellular bone marrows, low numbers of CD34 and functional hematopoietic progenitors, and high erythropoietin and thrombopoietin levels.16
Pulmonary Fibrosis.
Dyspnea and cough due to impaired gas exchange and reduced lung volume; bilateral fibrosis mainly at the lung bases by computed tomography scan; and a pathology of interstitial pneumonia, normal lung tissue alternating with honeycombing, fibrosis, interstitial inflammation, and collagen deposition define pulmonary fibrosis. Dyskeratosis congenita patients can develop pulmonary fibrosis, and lung complications occur after transplant. TERT and TERC mutant pedigrees often include cases of pulmonary fibrosis. These clinical associations led to the discovery of telomerase mutations as etiologic in up to 15% of cases of familial idiopathic pulmonary fibrosis.17,18 A smoking history also is common among affected individuals, suggesting a role of environmental factors in disease development. Telomeres are short in most patients with sporadic pulmonary fibrosis, and genome-wide association studies linked a TERT variant to idiopathic pulmonary fibrosis.19
Hepatic Disease.
As with pulmonary fibrosis, some patients with dyskeratosis congenita have liver abnormalities or fatal hepatic complications after bone marrow transplant. A few patients with pulmonary fibrosis and short telomeres also have cryptogenic hepatic cirrhosis, implicating telomere loss in both fibrotic processes.20 Relatives of patients with aplastic anemia and a telomerase mutation also may have liver disease that tracks to the mutation.21 The pathology is cirrhosis with inflammation or nodular regenerative hyperplasia, a leading cause of noncirrhotic portal hypertension. The association of nodular regenerative hyperplasia, aplastic anemia, and pulmonary fibrosis has been recognized in several families and is now understood to be secondary to telomere erosion and telomerase mutations.
Other Diseases.
Short-for-age telomeres have been observed in other human diseases, but with little or no genetic analysis, as, for example, in systemic sclerosis (in which pulmonary fibrosis is prominent). Accelerated telomere attrition has been correlated with atherosclerosis and a variety of poor cardiac outcomes; these associations are relatively weak, and they have been hypothesized to be due to telomere shortening secondary to chronic inflammation.
Telomeres and Cancer
Most cancers show gross derangement in chromosome numbers. Telomere attrition has been proposed as a mechanism for the loss or gain of chromosomes based on more than a century of cell and animal experiments.22–24 When telomere maintenance is disrupted in yeast, the few cells that escape senescence show chromosome abnormalities, end-to-end fusions, and consequent formation of dicentric and circularized chromosomes. In the absence of telomerase, genetic lesions increase due to terminal chromosome deletions and repeated cycles of break–fusion–bridge rearrangements. In “knock-out” mice that lack the RNA template component of telomerase, telomeres shorten progressively with each generation, producing chromosomal instability by end-to-end fusions. Most unstable cells are removed by apoptosis, but they can be rescued if DNA damage is not inadequately monitored: in mTERC–/– mice that also are deficient in the tumor suppressor gene p53, a variety of carcinomas appear associated with nonreciprocal translocations, as seen in human cancers.25
In humans, telomere length has been linked to malignant transformation—to the onset of cancer—in several diseases. When telomeres were first noted to be short in colorectal cancer, telomere loss was speculated to contribute to tumorigenesis and genetic instability. Telomerase deficiency has been reported in the histologically normal mucosa of inflammatory bowel disease. Losses of chromosomes in nondysplastic tissue of ulcerative colitis patients was correlated with telomere shortening and associated with the appearance of anaphase bridges, especially in patients who progressed to cancer.26
The major risk factor for esophageal cancer is the chronic inflammation of Barrett's esophagus, the result of years of exposure to gastroesophageal reflux. Leukocyte telomere length at first presentation is inversely proportional to the risk of later esophageal cancer, hypothesized to reflect a genetic predisposition to repair with persistent oxidative stress.27 Short-for-age leukocyte telomere length has been implicated as a risk factor or prognostic biomarker for many other solid tumors. Accelerated telomere shortening accompanies chronic graft-versus-host disease in human stem cell transplant recipients.28
For telomere studies, hematologic malignancies offer the advantages that telomere length can be easily measured in cells of origin of the cancer, and telomerase is normally active in hematopoietic stem cells. In classic dyskeratosis congenita, there are markedly elevated risks of many tumors (overall, about 11-fold), especially for head and neck squamous cell carcinomas, skin and anorectal cancers, and acute myeloid leukemia.29 In acquired aplastic anemia, telomere length of leukocytes is the major predictor of malignant clonal evolution: a large proportion of patients who developed monosomy 7 myelodysplastic syndrome and acute myeloid leukemia were in the lowest quartile of telomere length when they first presented with bone marrow failure.30 In acute myeloid leukemia, TERT hypomorphic variants were found in about 8% of cases, almost always associated with cytogenetic abnormalities; these germline mutations reduced telomerase activity in vitro.31 Telomerase mutations appear to account for familial acute leukemia.32 Short telomeres have been associated with leukemic transformation in myelodysplasia,33,34 the development of secondary myelodysplasia and leukemia, after chemotherapy and autologous hematopoietic cell transplantation,35,36 and critically short telomeres of blast cells correlated with gain and loss of chromosomes.37
Many genome-wide association analyses have now implicated telomere maintenance in cancer by the finding of genetic polymorphisms in the TERT gene at higher frequency in cancer populations. The level of risk is lower than when individual diseases are studied or when patient populations are assessed serially as malignancy develops in the setting of inflammation. One particularly large genome-wide scan associated the TERT locus with five (of 16) cancers: (1) basal cell cancer of the skin, (2) cancer of the lung, (3) cancer of the bladder, (4) cancer of the prostate, and (5) cancer of the cervix; the overall risks were relatively small (1.12–1.21), but consistent. Similar statistical associations have been reproducible for lung cancer, glioblastoma, and renal cell carcinoma. Conversely, single nucleotide polymorphisms within TERT associate with relative resistance to melanoma and breast cancer.
Practical Implications
As mutations in the telomere complex genes can affect multiple organs, the family history of a patient with aplastic anemia should include specific queries concerning lung and liver disease in relatives. There should be attention to the possibility of multiorgan involvement in an individual patient as well. Anecdotal experience suggests that telomerase mutations predispose to unusual sensitivity to chemotherapy delivered in standard regimens. For diagnosis, telomere length of leukocytes, although not now widely available commercially, should serve as a relatively simple screening test.38 Finding a mutation in one individual has significance for other family members—to be tested, in assessing disease risk, in avoiding environmental exposures such as smoking and alcohol, and in selecting donors for stem cell transplant. However, there are not as yet precise genotype-phenotype correlations, nor is there agreement on nosology. In general, DKC1 gene mutations lead to the most severe telomere shortening, as well as to early, severe, and multiorgan manifestations of classical dyskeratosis congenita. TERT lesions show more variable penetrance and often manifest in adult life in a single tissue and without typical dyskeratotic features. In marrow failure patients, short telomeres are the major risk factor for malignancies. Dyskeratosis congenita patients have very high rates of oral cancers and acute myeloid leukemia, and adult aplastic anemia patients with shorter telomeres have the highest likelihood of transformation to myelodysplasia and leukemia. Androgens may have therapeutic benefit not only in dyskeratosis congenita, but also in other marrow failure conditions accompanied by telomere shortening.
Research Implications
Although genetic and functional links between telomere biology and human disease are now clear, there are many open questions. Whereas hematopoiesis is obviously susceptible to a telomere pathophysiology, lung and liver are not conventionally considered regenerative organs, nor are the putative target cells in these organs identified. Conversely, why is the gut spared? Penetrance and organ-specific effects of a mutation are highly variable, between and within families, and both genetic and environmental factors need to be defined that influence the effects of decreased telomere repair capability. For one example, sex hormone modulation of telomerase, physiologic and pharmacologic, has not been studied. Many subtle features of telomere biology may translate to clinical relevance, such as genetic regulation of the length of the telomeres of individual chromosomes, the particularly short telomeres of chromosome 17 or of T regulatory cells, and the biologic impact of telomere attrition in specific cells in complex interacting systems, such as those involving stroma and immune effectors.
In the clinic, telomeres may have dominant roles in many processes that are amenable to study. Telomere attrition's central role in the origin of cancer from inflammation (in aplastic anemia, Barrett's esophagus, and ulcerative colitis) needs independent confirmation and then generalization to analogous diseases: chronic hepatitis B infection and hepatocellular carcinoma, cystitis and bladder cancer, chronic graft-versus-host disease, and cancer. In hematology and oncology, telomere length and telomerase genetics should be assessed in the risk of immediate, unexpected prolonged cytopenias and of late secondary malignancy after chemotherapy. Therapeutic use of sex hormones to modulate telomerase, especially in hematologic patients with iatrogenic gonad functional insufficiency, offers the possibility of prophylaxis against chromosomal instability and its dire consequences. Other factors that affect telomere biology, and other activities of telomerase besides telomere synthesis, can be examined using current in vitro systems. Finally, the marked difference between telomeres in the mouse and human, perhaps the result of years and generation of selection for breeding ability, suggests retrospective examination of complex murine model systems for their relevance to humans.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drug use: Male hormones and rabbit ATG for treatment of aplastic anemia.
Correspondence
Neal S. Young, MD, Chief, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Dr., Bldg. 10-CRC 3–5140, MSC-1202, Bethesda, MD 20892; Phone: (301) 496-5093; Fax: (301) 496-8396; e-mail: youngns@mail.nih.gov