Abstract
All cancers arise from complex interactions between aspects of the patient (host) biology and the environment. Once tumors arise, they frequently remain dependent on interactions with their microenvironment for their growth and proliferation. In this review, we examine the contributions of the host genetics and environmental exposures to the development of lymphoma. We will further examine the interactions of the tumor and the microenvironment that influence tumor growth and proliferation.
Most tumor cells cannot survive for long when taken outside their immediate environment. This indicates a level of dependence on secreted factors or direct cellular interactions with the microenvironment that provides survival signals. This dependence may arise from normal interactions of the normal cell counterpart or it might arise from new, abnormal interactions that arise from the tumor biology. The precancer host biology itself may have a role in either promoting tumor growth or permissive growth by either harboring polymorphisms that, in the right context, promote tumor growth, or through behaviors that increase exposure to elements that promote carcinogenesis.
Emerging technologies have provided new insights into the biology of a number of lymphomas. These technologies include microarrays that have enabled gene expression profiling to define the role of malignant and nonmalignant cells in the biology of lymphomas.
Herein, we review the known contribution of the tumor microenvironment and host biology to the risk of developing lymphoma and the role of inherited and acquired host factors in disease progression and prognosis in patients with lymphoma. We will examine the tumor “macro” environment (ie, the biology of the patient [host]) and the tumor “micro” environment that contribute to the observed malignant phenotype.
Role of Host Factors
The Most Common Acquired Risk Factor: HIV-AIDS
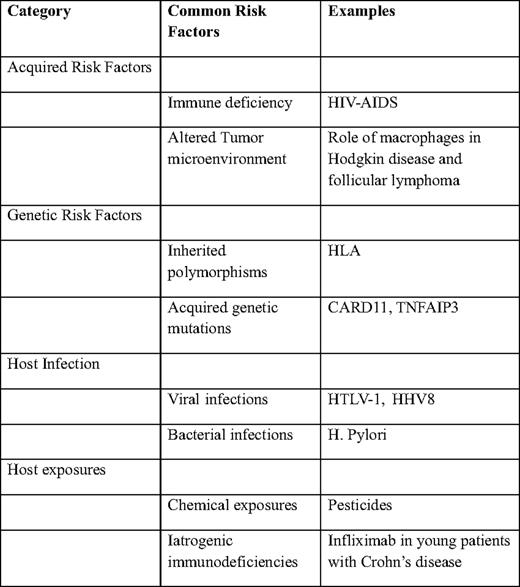
Diseases that impair cellular immunity significantly predispose to the development of lymphomas. HIV-AIDS (human immunodeficiency virus-acquired immune deficiency syndrome) is the most common cause of acquired immune deficiency in the world and thus the most important risk factor for the development of lymphomas (Table 1).
Although it clear that lower CD4 counts are associated with a higher risk of developing lymphoma, the advent of highly active antiretroviral therapy (HAART) appears to have significantly reduced the risk for development of lymphoma.1–3 In spite of the advent of HAART, the relative risk of developing lymphomas remains significantly elevated, compared with the HIV-seronegative population.4
Because the germinal centers of lymph nodes represent sites of primary interactions between CD4 T cells and B cells, it is not surprising that the majority of HIV-related lymphomas originate from germinal center B cells or postgerminal center B cells. The specificity of the T- and B-cell interactions and its role in the development of lymphomas is supported by the findings that HIV patients who have the CCR5–32 deletion that affects the ability of the HIV virus to infect T cells have a three-fold lower risk of developing AIDS-related lymphoma.5
There are three main groups of HIV-related lymphomas6 : (1) systemic non-Hodgkin lymphomas (NHLs) account for about 80% of the cases and include diffuse, large B-cell lymphoma (DLBCL), Burkitt lymphoma, Hodgkin disease, and plasmablastic lymphoma; (2) central nervous system (CNS) lymphomas account for 15% of HIV-related lymphomas, and the risk of CNS lymphomas is at least 1000-fold higher in patients with HIV7 ; and (3) primary effusion lymphomas form the last category of HIV-related lymphomas—the KSHV virus is strongly implicated in the development of these tumors.
The prognostic impact of HIV seropositivity on the prognosis of patients with NHL is uncertain. In general, these tumors are treated using the same regimens as HIV-seronegative patients.
Other Immune Deficiencies
Similar to HIV-AIDS, the risk of lymphoma in patients with common variably immune deficiency (CVID) is significantly higher.8 The lifetime risk of development of lymphoma in patients with CVID is estimated at about 8%.9 Lymphoma is also associated with other forms of immune deficiencies, including ataxia telangiectasia,10 Wiscott-Aldrich syndrome,11 severe combined immune deficiency, and X-linked lymphoproliferative disease.12 Organ transplantation, including hematopoietic stem cell transplantation, is associated with 10- to 100-fold increased risk for the development of lymphoma.
Thus, clinicians must maintain a high index of suspicion for the development of NHLs in patients with both acquired and genetic forms of immune deficiency.
Genetic Risk Factors
Although the genetic risk of lymphomas has not been fully characterized, there is a clear familial association with a number of NHLs.13 Thus, it seems likely that both inherited and acquired genetic variations play a role in the development of lymphoma.
Several studies have investigated the role of variation in specific genes and the development of lymphoma. A study of single nucleotide polymorphisms (SNPs) in 44 candidate immune genes in patients with DLBCL14 revealed a potential role for inherited genetic variation in tumor progression after treatment with standard chemotherapy. In particular, a combination of polymorphisms in four genes—interleukin (IL) A, IL8RB, IL4R, and tumor necrosis factor (TNF)—were all associated with overall survival in a multivariate model. These results were independent of the International Prognostic Index, as well as the known molecular subgroups of DLBCL, suggesting that these inherited genetic variants contribute independently to the biology of these tumors. Similar work has identified a potential role for SNPs in the genes encoding IL8, IL2, IL12B, and IL1RN in patients with follicular lymphoma (FL).15 Other work has identified polymorphisms in several immunoregulatory genes16 and HLA17 as potential contributing factors in patients with lymphoma.
Emerging studies also point to acquired genetic mutations in selected genes in the pathogenesis of lymphomas. Variation in a number of genes, including CARD11,18 A20,19 and EZH220 have been described as being important in the biology of NHLs. Whether these occur in inherited fashion and whether they potentially alter the risk of developing lymphoma is currently unknown.
It must be noted that the relative risk of developing NHL based on currently known genetic variants is fairly modest (odds ratios usually less than 1.5). Thus, the major determinant of genetic risk of lymphomas is currently unknown. Thus, most of the work in identifying genetic variation associated with the development of NHLs has been done on a gene-by-gene basis. The advent of massively parallel sequencing technologies has the potential to transform our understanding of the genetic basis of lymphoma.
Host Infections
A number of different viruses have been associated with the development of lymphomas.21 These include the Epstein-Barr virus, the human T-cell lymphotropic virus-1, and human herpes virus-8 that have been associated with Burkitt lymphoma, T-cell lymphoma-leukemia, and primary effusion lymphoma, respectively. In addition, hepatitis C and, possibly, hepatitis B viruses may also have a role in the development of lymphomas.
Several bacterial infections have been strongly associated with various lymphoma types, including Helicobacter pylori (gastric lymphoma), Campylobacter jejuni (small bowel lymphoma),22 Chlamydophila psittaci (ocular adnexal lymphoma), and Mycobacterium tuberculosis (pyothorax-associated lymphoma).
On one hand, the primary mechanism by which most viruses play a role in the development of lymphomas is by introducing alterations in the genome through the incorporation of viral genes. On the other hand, bacterial infections may induce a state of chronic inflammation that may be permissive to the development of tumors (see below).
Host Exposures
The role for environmental exposures in lymphomas is not well defined. A number of agents have been implicated in the development of lymphomas, including phenytoin, dioxins (eg, Agent Orange), pesticides, and ionizing radiation.
A growing recognition of the increased incidence of lymphoproliferative disorders in the setting of immune suppressive medications led to the introduction of “iatrogenic immunodeficiency-associated lymphoproliferative disorders” as a separate category in the latest World Health Organization classification of lymphoid neoplasms.23 The incidence of these disorders is not well characterized, and the precise relationship with the underlying disorder and immunosuppressive agent remains poorly understood. On the one hand, early observations suggest an association between the use of methotrexate in autoimmune diseases and the development of DLBCL, Hodgkin disease, and other lymphoproliferative disorders.24 On the other hand, the use of infliximab in young patients with Crohn's disease is associated with the development of hepatosplenic T-cell lymphoma.25 Similarly, increased risks of developing lymphoproliferative disorders have been described with the use of other TNF-α antagonists, including adalimumab and etanercept.24 The increasing use of these agents in patients with a variety of autoimmune diseases means that this group of disorders is likely to become an increasingly important cause of lymphomas, necessitating better methods of disease surveillance and better characterization of the mechanisms underlying the increased risk of lymphomagenesis.
The role of pesticides in the development of lymphomas was recently explored in a study that found an accumulation of t(14;18)+ cells in exposed patients.26 Because t(14;18) is the defining lesion of FL and a subset of DLBCL, this work shows a potential link between environmental exposure and genetic alterations that might culminate in the development of lymphoma.
Role of the Tumor Microenvironment
Inflammation and Lymphoma
The nexus between inflammation and cancer was first noted by Rudolf Virchow over a century ago.27 There are two major aspects that promote the formation of malignancy in the inflammatory microenvironment: (1) abnormal accumulation of cells and (2) accumulation of growth-promoting cytokines.
A prominent increase in the numbers of tumor-associated macrophages is a feature of many malignancies, including lung cancer, breast cancer, and lymphomas.28 Higher number of macrophages in the tumor biopsy has been a marker of poor prognosis in these tumors. In addition to their immune function, macrophages mediate tissue repair and wound healing by playing a key role in epithelial migration, matrix modeling, and angiogenesis. These same functions are also important in the progression of tumors. The parallels between wound healing and tumor progression have been examined using gene expression profiling.29 A molecular signature reflecting wound healing has been associated with poorer prognosis in a number of different cancers.30 The association between high numbers of macrophages and poor prognosis suggests that the normal functions of macrophages may be subverted by malignancies in a way that promotes tumor progression and metastasis.
The secretion of proinflammatory cytokines is also a prominent feature of a number of tumors. For instance, IL6 is a cytokine that is associated with inflammation and is known to promote the growth of multiple myeloma cells, as well as resistance to therapy.31 Higher IL6 levels are associated with increased risk of Hodgkin lymphoma32 and poorer prognosis in NHL.33
Inflammatory cytokines, in turn, lead to the induction of chemoattractant cytokines that recruit lymphocytes to sites of inflammation. Thus, the presence of a site of chronic inflammation can recruit a number of different cell types, with significant downstream effects. For instance, MALT (mucosa-associated lymphoid tissue) lymphomas arise in the setting of chronic gastritis caused by H. pylori infection. One of the chemokines induced by H. pylori is BCA-1,34 which recruits B cells to the site of infection where they can become targets for malignant transformation.
Thus, a chronic inflammatory state, whether induced by infection such as H. pylori, or through autoimmune processes (ie, Sjogren's syndrome, rheumatoid arthritis, systemic lupus erythematosus, or Hashimoto's thryoiditis) can predispose to the eventual development of lymphomas.
Discerning the Role of the Microenvironment in Lymphoma
The tumor microenvironment is defined as those nonmalignant cells that are closely associated with tumor cells in space and time. The tumor microenvironment can comprise diverse cell types, ranging from immune cells, endothelial cells, and normal cells of other lineages. Gene expression profiling has provided a new tool for distinguishing different components of the tumor microenvironment and its application has resulted in new insights into the role of the microenvironment in a number of different lymphomas.
For instance, in FL, a number of studies have demonstrated a role for the microenvironment.35–37 In particular, two immune signatures—(1) immune response-1 reflecting expression from T cells and (2) immune response-2 reflecting expression from macrophages38 —have been shown to be associated with survival. A multivariate model based on the expression levels of these two gene expression signatures was highly predictive of long-term outcome in FL patients. These results suggest that interactions between the tumor and the microenvironment are established early in the course of the disease and may determine long-term outcomes in the disease. In FL, the association of erythrocyte sedimentation rate (ESR) and survival has been established by several studies.39,40 It is unclear whether elevated ESR and other serum markers of inflammation are associated with a higher expression of the macrophage-associated immune response-2 signature. Determinants of these immune response signatures remain unknown.
The role of the microenvironment is also important in aggressive lymphomas. In DLBCL, a combination of two separate gene expression signatures derived from nonmalignant cells, termed stromal-1 and stromal-2, were strongly associated with survival in patients treated with standard R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone) chemotherapy regimens.41 The stromal-1 signature was found to reflect deposition of extracellular matrix and associated with favorable outcome. Yet, the stromal-2 signature was found to reflect tumor blood vessel density and a poorer outcome.
In Hodgkin lymphoma, the malignant Reed-Sternberg cells are considerably outnumbered by nonmalignant cells in the tumor bed. Thus, studying the biology of these tumors has been challenging. Gene expression profiling enabled the identification of the number of macrophages in the tumor biopsy as a major determinant of prognosis in these patients.42 Consistent with their described role in other malignancies, the presence of a high number of macrophages in the tumor biopsy was associated with poorer outcome.
These studies in diverse lymphoma types are likely to represent the vanguard of other studies that will likely reveal a role for the microenvironment in other lymphoma types.
Conclusions
The nature of the microenvironment in lymphomas clearly deserves further exploration. Major unanswered questions remain regarding the relative contributions of the tumor and the microenvironment to the malignant phenotype. For instance, it is possible that inherited genetic polymorphisms and/or environmental exposure to pathogens might modulate the risk of acquiring the disease, as well as the nature of the tumor infiltrating cells and the microenvironment. Alternatively, the genetic characteristics of the malignant cells may be the primary determinant of the tumor microenvironment.43
Genetics remains the common denominator of tumor biology. Lymphomas likely arise from a confluence of inherited genetic variation and environmental exposures, including infections and the presence of inflammatory conditions. Each of these factors is likely to leave its molecular fingerprints on the genome. The advent of new genetic tools (ie, massively parallel sequencing) provides new opportunities for the molecular definition of host and tumor. A complete molecular definition of lymphomas will provide new opportunities for defining the nexus that exists between the host factors and tumor. Agents that break that nexus will provide a new class of therapeutic opportunities in lymphomas where such options are urgently needed.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drug use: None disclosed.
Correspondence
Sandeep S. Dave, MD, MBA, Department of Medicine/Medical Oncology, Duke University Medical Center, #3382, 101 Science Drive, Durham, NC 27708; Phone: (919) 681-1922; Fax: (919) 684-4777; e-mail: sandeep.dave@duke.edu