Abstract
Conventional allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment option for various hematological diseases due, in part to high-dose conditioning and, in part, to graft-versus-tumor effects. Reduced-intensity or non-myeloablative conditioning regimens have relied mostly on graft-versus-tumor effects for disease control, and their advent has allowed relatively older and medically infirm patients to be offered allo-HCT. However, both HCT modalities have been associated with organ toxicities and graft-versus-host disease, resulting in substantial non-relapse mortality. It has become increasingly important to optimize pre-transplant risk assessment in order to improve HCT decision making and clinical trial assignments. Single-organ comorbidity involving liver, lung, heart, or kidney before HCT has been traditionally found to cause organ toxicity after HCT. Recent efforts have resulted in the advent of a weighted scoring system that could sensitively capture multiple-organ comorbidities prior to HCT. The HCT-comorbidity index (HCT-CI) has provided better prediction of HCT-related morbidity and mortality than other non-HCT-specific indices. Subsequent studies, with the exception of a few studies with modest numbers of patients, have confirmed the prognostic importance of the HCT-CI. Further, the HCT-CI has been consolidated with various disease-specific and patient-specific risk factors to refine assignments of patients to the appropriate HCT setting. Ongoing studies are addressing prospective validation of the HCT-CI, furthering our understanding of biological aging, and enhancing the applicability of the HCT-CI comorbidity coding. Future knowledge of the impacts of multiple comorbidities on post-HCT toxicities might guide new prophylactic and therapeutic interventions to lessen the procedure's mortality.
Introduction
A balanced risk-benefit approach to hematopoietic cell transplantation (HCT) is the key for maximized chances of cure with acceptable quality of life for patients with advanced hematologic malignancies. When considering efficacy, high-intensity conditioning regimens could eradicate the underlying malignancy that was previously refractory to all other conventional therapeutics. Infused allografts rescue patients from the lethal toxicity of conditioning regimens, but most importantly they serve as a lifelong consolidation treatment, via the power of graft-versus-tumor effects, preventing disease relapse. In the setting of non-myeloablative (NMA)-HCT, the donor immune system is relied on for both eradicating the underlying malignancy and preventing relapse. In addition, the new healthy grafts could act as a platform for better tolerance of maintenance immuno-chemotherapy after HCT.
In regard to safety, HCT can have four complex impacts on organ functions. First, conventional high-dose HCT preparative regimens have been known for causing serious toxicities to organs such as the gut, liver, kidney, heart, and lung. Organ toxicities can still be observed after reduced-intensity conditioning (RIC) or NMA regimens.1,2 This is mainly because these regimens are almost exclusively offered to older and medically infirm patients who would have been ineligible for conventional HCT. Second, the myeloablation and/or immunosuppression caused by conditioning and post-grafting immunosuppressive treatments could set the stage for serious infections.3,4 Third, the donor T cells, and also to some extent B cells, are responsible for a series of acute and chronic graft-versus-host syndromes.5–7 Finally, total body irradiation, chemotherapy, post-grafting prophylactic and therapeutic immunosuppression, and graft-versus-host disease (GVHD) could increase risks of long-term morbidity8,9 and secondary malignancy.10 All four impacts could result in increased mortality and impaired quality of life after HCT.
A roadmap for maximization of the benefits of HCT would require novel approaches for both improving tumor control and lessening morbidity and mortality after HCT. The latter is the main focus of this article, and it requires a better understanding of the biology and extents of interactions between HCT-associated interventions and patient-specific risk factors. This would allow for accurate pre-transplant benefit-risk assessment leading to assignments of patients to the most tolerable modality of HCT.
Chronological age has been long used as the main HCT prognostic variable, and arbitrary age cutoffs have been set up as exclusion criteria for high-dose conditioning regimens. However, age per se is a poor predictor of HCT outcomes,11 probably due to a lack of data on organ malfunctions in most previous reports. In addition, protocol exclusion criteria bias any assessment of the influence of aging, and recent reports failed to show any impacts of chronological age on allogeneic HCT (allo-HCT) outcomes.12–14 Biological aging is hypothetically composed of three parameters: age, physical function, and organ comorbidities. Indeed, among 427 cancer patients of whom over 50% had either leukemia or lymphoma, age, functional impairment, and comorbidities were found to independently predict patient survival regardless of the kind of cancer.15
Among cancer patients, increasing comorbidities have been associated with offering no or less-than-ideal treatment, exclusion from clinical trials, increasing post-treatment toxicities and mortality, and impaired quality of life. These findings were confirmed in patients with non-Hodgkin's lymphoma receiving conventional treatment.16 Statistically, comorbidities could either be confounders, limiting the internal validity, or effect modifiers, affecting the internal and external validity of studies.17
It has become increasingly important to comprehensively study pre-HCT comorbidities and their impacts on HCT outcomes given the aforementioned results, as well as: 1) the aging of Western populations; 2) increasing numbers and severity of comorbidities among the older population18 ; 3) relatively high median age (65–70 years) at diagnosis of most hematologic malignancies19 ; 4) the advent of RIC/NMA regimens, which has increased the age cutoff for HCT to the eighth decade of life13,14 ; and 5) the need for weighted tools to better direct patients to the appropriate HCT conditioning regimen.20 This review discusses recent advances in understanding the roles of multiple comorbidities in the setting of HCT.
Historical Studies on Impacts of Single-Organ Comorbidity on HCT Outcomes
In the setting of HCT, adequate organ functions are patients' reserves to withstand the harshness of chemo-radiotherapy, to process different medications and large volumes of fluids, and to tolerate serious infections and the harmful effects of GVHD. Several investigators have studied correlations between pre-transplant organ malfunctions and post-HCT morbidity and mortality. Most if not all of the earlier studies have focused on single-organ malfunction, represented by laboratory findings, and its impact on the same organ toxicity after HCT.
Conflicting data have been reported on the prognostic importance of pre-transplant reduced left-ventricular ejection fraction. One study failed to demonstrate any impacts on severe post-HCT cardiac toxicities,21 another was limited by the rarity of post-HCT cardiac events,22 while in a third the impacts were limited to cardiac toxicities and no information on mortality was given.23 In contrast, abnormalities of pulmonary-function tests have been consistently found to affect HCT mortality. Low pre-transplant diffusion capacity of the lung for carbon monoxide (DLCO) was found to be associated with post-HCT death, but not due to respiratory failure,24 while low forced expiratory volume in 1-second (FEV1) was a risk factor for cytomegalovirus-associated interstitial pneumonitis.25 Recent studies have identified the two abnormalities to be the most uncorrelated pulmonary-function test variables,26 to predict mortality26,27 and decline in lung functions after HCT,28 and to differentiate between the insults of regimens with different intensities.29
Abnormal liver (primarily elevated serum transaminases) and renal (primarily elevated serum creatinine) function tests have been studied for prediction of early mortality.27 Post-HCT sinusoidal obstruction syndrome and liver injury have been frequently seen among patients with pre-HCT elevated transaminase values or chronic hepatitis.30–32 Elevated pre-HCT serum creatinine was found to be a risk factor for post-HCT acute renal failure and mortality.27,33 Elevated serum creatinine could also be a limitation against efficient dosing of calcineurin inhibitors for the prevention of GVHD. Rare other reports have investigated the impacts of other single comorbidities. Depression, for example, was found to increase the risk of dying within 12 months after HCT by 3-fold.34 Still, there has been no known validated systematic method to integrate all these prognostic information into a decision-making algorithm for HCT. Further, as hinted earlier, most of the aforementioned studies on single-organ comorbidity did not correct for the impacts of other comorbidities.
Early Efforts to Summate Comorbidities into a Single Scoring System
It would be counterintuitive to think that single-organ comorbidity would affect morbidity and mortality after HCT in isolation from other ongoing organ malfunctions. However, until 2003,35 there was almost no effort made to evaluate the distribution of comorbidities among HCT recipients, to investigate how increasing numbers and/or severity of comorbidities affect HCT outcomes, or to include them in designing clinical trials. To better understand indications of NMA allo-HCT from unrelated donors, investigators from Fred Hutchinson Cancer Research Center (FHCRC) carried out a study to assess clinical pre-transplant differences and compare toxicities, GVHD, and non-relapse mortality (NRM) between consecutive and concurrent NMA (n = 60) and myeloablative patients (n = 74). Investigators used the Charlson Comorbidity Index (CCI) as a measure of comorbidities and as a novel parameter for pre-transplant clinical assessment. The CCI included 19 comorbidities that have been weighted on the basis of their strengths of associations with mortality among patients with general medical conditions.36 It is the most widely used comorbidity index in predicting mortality risks in various solid malignancies. The study results were presented at the ASH annual meeting,35 and were later published,1 showing that comorbidities, as scored by the CCI, were the only independent factor predicting grade IV non-hematologic toxicities at 100 days and 1 year after NRM. The CCI scores could be used to compare and stratify NMA and myeloablative patients into low- and high-risk groups for outcomes. The same investigators showed in a parallel study that the CCI was important in predicting NRM among related recipients,2 but with a weaker magnitude of association than in the unrelated study. Later, FHCRC investigators found that CCI scores of 0, 1, and ≥ 2 predicted 2-year NRM of 7%, 31%, and 46%, and overall survival of 78%, 49%, and 33%, respectively, among patients with chronic lymphocytic leukemia (CLL) treated with NMA allo-HCT.37
Two other groups of investigators have started addressing the importance of comorbidity for HCT, and presented their data at the ASH annual meeting. Investigators from M.D. Anderson Cancer Center (MDACC)38 found among 51 patients with acute myeloid leukemia (AML) or myelodysplasia (MDS) that age-adapted CCI39 scores of 0 to 2 predicted lessened NRM (0% vs. 18%; p = 0.02) and improved overall survival (73% vs. 53%; p = 0.04) compared with CCI scores of > 2. Pollyea et al. presented data showing the Kaplan-Feinstein Index to be a more sensitive indicator of comorbidity than the CCI among 81 patients given RIC allo-HCT.40 In a multivariate analysis, patient age and performance status were predictors for NRM, while comorbidity scores and performance status were predictors for survival.
The HCT-Specific Comorbidity Index
The CCI was found to be of limited sensitivity in detecting comorbidities among HCT recipients, with only 12% of FHCRC myeloablative patients acquiring scores of ≥ 1.1 This was thought to be due to the fact that the CCI was not developed in the HCT setting, and therefore it lacked definitions of some common comorbidities such as infections, while it included crude definitions for others such as hepatic and pulmonary comorbidities. Clearly, lack of sensitivity would be a major limitation against using the CCI in clinical HCT protocols and studies.
To standardize and generalize the assessment of multiple comorbidities in the HCT setting, the FHCRC investigators modified the CCI to be better fitted for the transplant population.41 A new retrospective study included 1055 consecutive patients with various hematologic diseases who were either given NMA, 2 GY total body irradiation (TBI) ± fludarabine (Flu) (n = 294) or myeloablative cyclophosphamide (Cy)/12 Gy TBI or busulfan (Bu)/Cy (n = 761) conditioning followed by allo-HCT from related or unrelated donors. Patients were randomly distributed into training (n = 708) and validation (n = 347) sets. In the training set, investigators introduced three major conceptual changes to the CCI: 1) all comorbidities in the patients' past medical history or abnormal laboratory values immediately before start of conditioning were included41 ; 2) definitions of cardiac, pulmonary, hepatic, and renal comorbidities were refined by relying mainly on objective values of laboratory and organ function tests; and 3) new adjusted hazard ratios for NRM were calculated to constitute the foundation of the new weighted model. Scores were weighted for the impacts of all comorbidities, disease risk, conditioning intensity, and age. The investigators introduced the use of progressive impairments of left-ventricular ejection fraction for cardiac, DLco/FEV1 for pulmonary, and progressive elevations of bilirubin and transaminases for hepatic comorbidities. All of these parameters have been used to grade treatment toxicities per the Common Toxicity Criteria (CTC) of the National Cancer Institute (NCI). Further, new cutoffs for serum creatinine levels were set to better define renal comorbidity. The analyses included 24 single comorbidities, and three of those, pulmonary, hepatic, and renal, had two or three levels of severity. Adjusted hazard ratios from Cox proportional hazard models identified 17 comorbidities to be independently predictive for NRM. Two comorbidities, pulmonary and hepatic, were represented twice based on severity, and three new comorbidities were added to the historical CCI: morbid obesity (as defined by the body mass index), infections, and psychiatric disturbances (Table 1).
The hematopoietic cell transplant-comorbidity index (HCT-CI)

*Diagnosed at any time in the patient's past history; §Values detected at the closest time prior to start of conditioning regimen.
ULN, upper limit of normal; DLco, diffusion capacity of carbon monoxide; FEV1, forced expiratory volume in one second; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The design of the HCT-CI had the strength of including: 1) a fairly large sample size; 2) consecutively transplanted patients, as a good representation of the general population of patients transplanted at different institutions; and 3) a wide spectrum of diagnoses and conditioning regimens in the multivariate models. The increasing weights of the HCT-CI were meant to capture a general trend for increases in risks of NRM (scores 0, 1, 2, 3, and ≥ 4 predicted NRM of 9%, 14%, 27%, 41%, and 43%, respectively), the range of which would vary based on the intensity of conditioning regimens, risks of disease status, and other factors. In the validation set, and to facilitate comparisons, HCT-CI scores were collapsed into three risk groups 0, 1 to 2, and ≥ 3, which statistically significantly predicted NRM (14%, 21%, and 41%) and overall survival (71%, 60%, and 34%), respectively. By comparison, CCI scores of 0, 1, and ≥ 2 were associated with no differences in NRM (23%, 29%, and 25%, respectively). Compared with the CCI, the HCT-CI had higher c-statistics for 1-year NRM (0.692 vs. 0.546) and overall survival (0.661 vs. 0.561).
Could the HCT-CI Be Used in Optimizing Transplant Indications for a Given Hematologic Malignancy?
The HCT-CI summated the relevant pre-transplant organ comorbidities as a single risk factor, in which weights of comorbidity burdens were adjusted for the complex impacts of disease risks and conditioning intensities. This has created a new path for accurate risk-benefit ratio assessment before HCT.
The HCT-CI and Other Traditional Risk Factors for HCT Outcomes
One of the main advantages of summating patient comorbidities into the HCT-CI is the ability to consolidate this index with one or more risk factors for HCT outcomes to strengthen both statistical analyses for clinical prognostication and decision making for HCT choices. The HCT-CI has been combined with disease-specific factors such as disease status to better risk-stratify patients with AML or MDS before allo-HCT,42 lymph node size to refine outcomes of CLL patients after allo-HCT,43 or chemosensitivity to optimize indications of autologous HCT for lymphoma.44 It has also been combined with Karnofsky performance status (KPS) to classify NMA-HCT recipients into four risk groups for NRM and overall survival.45 Further, the HCT-CI and C-reactive protein (CRP) were recently used together to rationalize the indications of allo-HCT for patients with chronic myelocytic leukemia (CML) who failed imatinib therapy.46
Allo-HCT for Patients with AML/MDS
FHCRC investigators reported 2-year overall survival of 70% and 78% among AML/MDS patients with HCT-CI scores of 0 to 2 and low-risk diseases following NMA- and myeloablative HCT, respectively.42 The figures for patients with high-risk diseases were 57% and 50%, respectively. Therefore, AML/MDS patients with low comorbidity burden could be targeted in prospective, randomized studies to determine the role of conditioning intensity. In contrast, patients with HCT-CI scores of ≥ 3 had relatively inferior survival rates in the FHCRC study, particularly those with high-risk diseases (overall survival of 29% and 24%, respectively). This was mainly due to relatively more frequent relapse (49%) among NMA patients and more frequent NRM (46%) among myeloablative patients. Interestingly, HCT-CI predicted not only increased NRM but also increased relapse rates. This was the first report to point out a possible biological link between pre-HCT comorbidities and leukemia aggressiveness. Further investigations are warranted.
Investigators from United Kingdom and the Netherlands confirmed the inferior overall survival of 32% among 128 patients with AML/MDS and HCT-CI scores of ≥ 3 after alemtuzumab-based RIC HCT.47 Patients with HCT-CI scores of 0 and 1 to 2 had an overall survival of 69% and 39%, respectively. In the multivariate analysis, both the HCT-CI scores and disease status at HCT were the strongest predictors of increased NRM and poor survivals, similar to the FHCRC study, while chronological age was of no prognostic value. Results of both studies showed that patients with high comorbidity and disease burden might benefit from enrolling in clinical trials investigating dose-escalating conditioning regimens48,49 or exploring novel maintenance drugs after NMA regimens.50 Investigators from the European Group of Blood and Marrow Transplantation (EBMT) reported 2-year NRMs of 9%, 15%, 18%, and 31%, respectively, for HCT-CI scores of 0, 1, 2, and ≥ 3 among AML patients in a first complete remission given various reduced-intensity regimens.51 Boehm et al. confirmed the inferior survival of MDS patients with high HCT-CI scores after conditioning regimens of different intensities.52 In a different study, patients with CML, who were treated mostly with conventional allo-HCT and had HCT-CI scores of ≥ 3, were found to have overall survival of 15% compared with 54% for those with scores of 0 to 2.53
In aggregate, patients with low-risk comorbidities might be better served by enrolling in randomized studies between myeloablative and RIC/NMA regimens, while patients with high HCT-CI scores could benefit from novel conditioning regimens that target leukemia while avoiding the lethal toxicities.
HCT for Patients with CLL, Lymphoma, or Multiple Myeloma
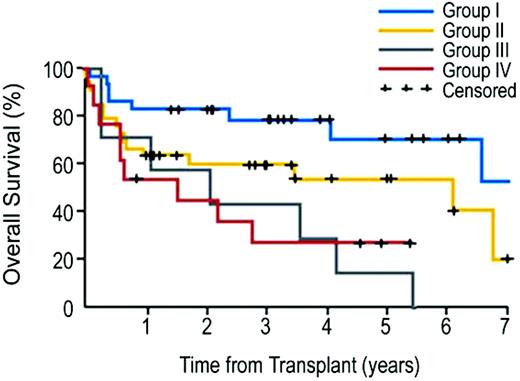
Conventional allo-HCT is notorious for causing high NRM (up to 50%) among patients with CLL or lymphoma.54,55 In the era of the HCT-CI, FHCRC investigators have shown the relatively high NRM (50%) after myeloablative HCT to be limited to patients with relevant comorbidities per the HCT-CI (scores > 0) resulting in overall survival of 35%. By comparison, more favorable NRM (28%) and overall survival (47%) rates were reported among recipients of 2 Gy TBI ± Flu and allo-HCT with HCT-CI scores > 0.56 Of particular interest, NRM was not different after the two conditioning regimens when patients had no comorbidities per the HCT-CI (15% and 18%, respectively). Not surprisingly, conditioning intensity and HCT-CI were the main influential factors for outcome prediction in the multivariate models. No differences could be detected in relapse rates after myeloablative or NMA regimens regardless of disease histology, suggesting a minimal role of conditioning intensity in this patient cohort. In another study of 82 CLL patients treated with NMA-HCT, the HCT-CI was combined with an adverse disease-feature, namely lymph node size over 5 cm, to stratify patients into four risk groups (Figure 1).43 Patients with or without comorbidities who had lymph node size under 5 cm achieved 5-year overall survival of 60% and 78%, respectively, while those with lymph node size ≥5 cm had overall survival of 27% and 43%, respectively.
Kaplan-Meier probabilities of survival among patients with advanced CLL who were treated with allo-NMA-HCT as stratified into four risk groups on the basis of consolidated HCT-CI scores and lymph node diameter. Group I included patients who had no comorbidities and who had lymphadenopathy of < 5 cm (n = 28); group II, patients with comorbidities only (n = 34); group III, patients with lymphadenopathy of ≥ 5 cm only (n = 7); and group IV, patients with both comorbidities and lymphadenopathy of ≥ 5 cm (n = 13). Five-year survival rates were 78%, 60%, 43%, 27% for risk groups I, II, III, and IV, respectively. (From Sorror et al., 2008.43 Reprinted with permission. ©2008 American Society of Clinical Oncology. All rights reserved.)
Kaplan-Meier probabilities of survival among patients with advanced CLL who were treated with allo-NMA-HCT as stratified into four risk groups on the basis of consolidated HCT-CI scores and lymph node diameter. Group I included patients who had no comorbidities and who had lymphadenopathy of < 5 cm (n = 28); group II, patients with comorbidities only (n = 34); group III, patients with lymphadenopathy of ≥ 5 cm only (n = 7); and group IV, patients with both comorbidities and lymphadenopathy of ≥ 5 cm (n = 13). Five-year survival rates were 78%, 60%, 43%, 27% for risk groups I, II, III, and IV, respectively. (From Sorror et al., 2008.43 Reprinted with permission. ©2008 American Society of Clinical Oncology. All rights reserved.)
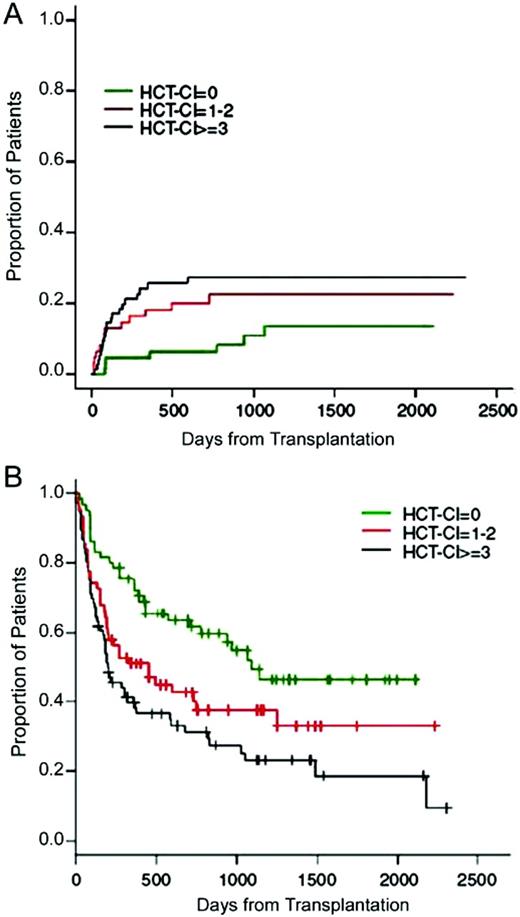
A study from Italy included 203 patients with non-Hodgkin's lymphoma, Hodgkin's lymphoma, or multiple myeloma treated with either 2-Gy TBI-based NMA or Flu-Cy-based RIC regimens and allo-HCT.57 HCT-CI scores of 0 were associated with a favorable overall survival of 87% at 2 years, while scores of 1 to 2 and ≥ 3 were associated with overall survival of 51% and 49%, respectively. No statistically significant differences could be detected in overall survival between patients with scores of 1 to 2 or ≥ 3, suggesting equivocal tolerability of those patients to the RIC HCT. In multivariate analyses, HCT-CI and KPS were independent predictors of overall survival, while only HCT-CI predicted progression-free survival (Figure 2). Among 63 non-Hodgkin's lymphoma patients conditioned with the Flu/Cy regimen followed by allo-HCT, Pollack et al. confirmed the higher NRM (36.8%) associated with HCT-CI scores of ≥ 3 compared with 13.6% with scores of 0 to 2.58
(A) Cumulative incidence of non-relapse mortality (p = 0.04) and (B) progression-free survival (p < 0.001) as stratified by pretransplant HCT-CI among patients with lymphoma, CLL, and multiple myeloma. (From Farina et al., 2009.57 Reprinted with permission. ©2009 Macmillan Publishers, Ltd.)
(A) Cumulative incidence of non-relapse mortality (p = 0.04) and (B) progression-free survival (p < 0.001) as stratified by pretransplant HCT-CI among patients with lymphoma, CLL, and multiple myeloma. (From Farina et al., 2009.57 Reprinted with permission. ©2009 Macmillan Publishers, Ltd.)
The FHCRC investigators presented data at the ASH annual meeting summarizing the impacts of comorbidities on autologous HCT outcomes among 273 patients with non-Hodgkin's lymphoma or Hodgkin's lymphoma.44 Patients with HCT-CI scores of 0 and 1 to 2 had similar NRM of 3% and 8%, suggesting equal tolerance to conditioning regimens. Patients with HCT-CI scores of 3 to 4 and ≥ 5 had NRM of 15% and 42%, respectively. The HCT-CI scores of ≥ 3, high lactate dehydrogenase, and chemo-resistance at HCT were independently associated with increased overall mortality. Patients who had the three unfavorable factors at HCT had median overall survival of 7.8 months.
From the aforementioned results, HCT-CI scores of more than 0 among CLL/lymphoma/multiple myeloma patients could be used as a criterion to favor NMA/RIC over conventional high-dose regimens. Autologous HCT might be more safely avoided in patients with HCT-CI scores of ≥ 5. Patients with adverse disease features (such as large lymph nodes or chemo-refractoriness) with no comorbidities might benefit from the intensity of the myeloablative HCT. Patients with adverse disease features and comorbidities might be better served by approaches combining NMA-allo-HCT with prior debulking autologous HCT,59 concurrent radiolabeled anti-CD20 monoclonal antibody,60 or proceeding disease-specific maintenance treatment.
Allo-HCT for Patients with CML
Investigators from the United Kingdom and Spain used the HCT-CI in combination with pre-HCT values of an inflammatory marker (CRP) to re-write HCT recommendations for patients with CML in the era of tyrosine-kinase inhibitors. First, they confirmed in a multivariate model the independent prognostic impact of the HCT-CI on NRM and overall survival among 271 CML patients who failed imatinib and were treated with Cy/TBI and allo-HCT. CRP levels of ≤ 9 ml/L also predicted relatively lessened NRM and better overall survival, and therefore investigators thought that both HCT-CI scores of 0 and CRP ≤ 9 ml/L would identify a group of CML patients with the most favorable outcome after allo-HCT. Patients with adverse comorbidities or high CRP levels might benefit from second-line tyrosine-kinase inhibitors.46
Impacts of Multiple Comorbidities on Post-HCT Organ Toxicities
Minimal results have been published so far to describe how the interaction between multiple comorbidities, represented by weighted scores, affect single-organ toxicity. FHCRC investigators have shown twice that increasing CCI and HCT-CI scores were associated with higher cumulative incidences of grade III and IV non-hematologic toxicities, as graded by the CTC of the NCI.1,45 Investigators from Canada found both the CCI and the HCT-CI scores of more than 0 to be associated with an increased number of organ systems with serious toxicity (at least grade 2 toxicity using the Seattle criteria61 ) and an increased total sum of toxicity grades for all organs, in addition to prolonged hospitalization after autologous HCT.56,62 FHCRC investigators have shown that CLL/lymphoma patients with no comorbidities spend less time in hospitals if the conditioning regimen for allo-HCT was of low intensity.56 Investigators from City of Hope found that patients with late post-HCT congestive heart failure more frequently had preceding hypertension, chronic renal insufficiency, chronic lung disease, and diabetes than those without. Further, preceding ≥ 2 comorbidities were associated with increased post-HCT congestive heart failure.63 A study from MDACC found increased incidence of post-HCT cardiac toxicities if patients had one of seven preceding cardiac comorbidities.64 Clearly, additional investigations are required to better predict and prevent organ toxicity based on preexisting multiple comorbidities.
Are Comorbidities and Performance Status Replaceable?
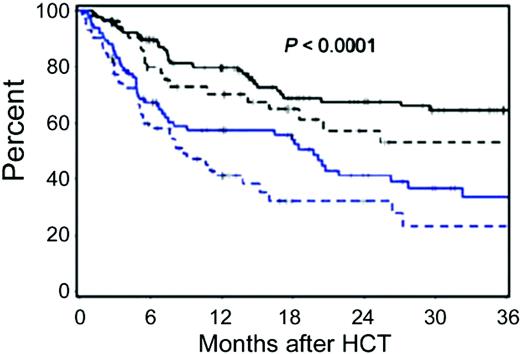
Comorbidity is the presence of one or more additional organ-specific malfunctions in the presence of a separate primary disease. Comorbidity impacts are sensitive to definitions, and this is where indices are needed to provide specific, valid, and objective reproducible definitions for comorbidities. Performance or functional status is a simple evaluation of the overall fitness of the patient that mostly cannot be separated from the impact of the primary disease. It is already known that comorbidity indices and performance status scales independently predict cancer treatment outcomes.65 FHCRC investigators have shown weak correlations between HCT-CI and KPS among 341 patients with hematologic malignancies given NMA-allo-HCT. KPS was assigned prospectively by clinicians and retrieved retrospectively by the study investigators. Both HCT-CI and KPS independently predicted NRM and overall survival but with greater predictive power by the former. Given the relatively lessened NRM after NMA-HCT, a refined risk-stratification model was proposed by consolidating the HCT-CI and the KPS. The consolidated tool stratified NMA patients into four risk groups with overall survival of 68%, 58%, 41%, and 32%, respectively, at 2 years (Figure 3).45 Two other studies found KPS to independently predict NRM to a greater extent than comorbidities.66,67 The former study used a non-HCT-specific comorbidity index, and both studies transformed some Eastern Cooperative Oncology Group scores to KPS percentages, which was previously suggested to be associated with a high level of error.68
Kaplan-Meier probabilities of survival among patients with hematologic malignancies treated with allo-NMA-HCT as stratified into four risk groups based on a consolidated HCT-CI and KPS scale. Group I (solid black line) includes patients with HCT-CI scores of 0 to 2 and a KPS of > 80%; group II (dotted black line) includes patients with HCT-CI scores of 0 to 2 and a KPS of ≤ 80%; group III (solid blue line) includes patients with HCT-CI scores of ≥ 3 and a KPS of >80%; group IV (dotted blue line) includes patients with HCT-CI scores of ≥ 3 and a KPS of ≤ 80%. Survival rates at 2 years were 68%, 58%, 41%, 32% for risk groups I, II, III, and IV, respectively. (From Sorror et al., 2008.45 Reprinted with permission. ©2008, Wiley InterScience.)
Kaplan-Meier probabilities of survival among patients with hematologic malignancies treated with allo-NMA-HCT as stratified into four risk groups based on a consolidated HCT-CI and KPS scale. Group I (solid black line) includes patients with HCT-CI scores of 0 to 2 and a KPS of > 80%; group II (dotted black line) includes patients with HCT-CI scores of 0 to 2 and a KPS of ≤ 80%; group III (solid blue line) includes patients with HCT-CI scores of ≥ 3 and a KPS of >80%; group IV (dotted blue line) includes patients with HCT-CI scores of ≥ 3 and a KPS of ≤ 80%. Survival rates at 2 years were 68%, 58%, 41%, 32% for risk groups I, II, III, and IV, respectively. (From Sorror et al., 2008.45 Reprinted with permission. ©2008, Wiley InterScience.)
KPS was originally designed to measure self-care, daily activities, and evidence of disease, rendering it both a patient-specific and a disease-specific risk factor. Even though it was shown that impaired performance status is linked to increasing NRM, multiple other studies have failed to show any prediction of NRM by performance status.69–72 Moreover, impaired performance status is strongly correlated with high-risk diseases, and its impact on overall survival was found to be exclusively due to increasing rates of relapse and relapse-related mortality.70–72 These conflicting results on the impacts of performance status could be due to, in part, lack of guidelines for accurate performance status assessment and, in part, physician tendency to assign 100% performance status to patients with no active disease or gross disability and to look only at observation and physical examination rather than activities and daily living.73
Nevertheless, there is now ample evidence in the HCT setting that comorbidities and performance status capture different levels of patients' health status. Both should be assessed for outcome prediction studies and clinical trial enrollments. Developing guidelines for performance status assignment and introducing concise geriatric assessment before HCT might improve our understanding of functional impairments at the time of HCT.
Validity and Reliability of the HCT-CT
FHCRC investigators initially reported results on the validity of the discriminative power of the HCT-CI.41 Later, FHCRC and MDACC investigators confirmed the ability of the HCT-CI, scored by independent investigators at each institution, to accurately predict NRM among AML patients. Comparable NRM could be seen among both populations when stratified by the HCT-CI scores.74 Further, while FHCRC patients had 71% versus 56% overall survival compared with MDACC patients, respectively, the hazard ratio was 0.98 after adjusting for differences in comorbidities, stressing on the importance of including comorbidity indices in comparing trial results.
The success of the HCT-CI to predict NRM and overall survival was further demonstrated by FHCRC investigators in a relatively large number of disease-specific reports.42–44,53,56,75,76 Other investigators confirmed in at least 12 studies the strong prediction of HCT outcomes by the HCT-CI among overall or specific hematologic malignancies.45,47,52,58,62,77–84 At least five other studies reported on the ability of the HCT-CI to provide good prediction of conventional treatment outcomes among patients with AML or MDS, which adds another layer to the generalizability of this index.85–88 One study found the HCT-CI to be a sole independent predictor of outcomes among patients receiving relative or cord blood grafts, but of less strength in smaller samples of subgroup analyses.89 Only four studies disagreed with the validity of the HCT-CI for outcome prediction.67,72,90,91 In two of these four studies, the interpretation of results has been seriously limited by the small sample of patients (N = 81)90 and number of events and the lack of significant amount of data (pulmonary-function tests) necessary for accurate assignment of comorbidity weights.91 The other two studies included data from about 150 patients each, who were transplanted over long period of time dating back to early 1990s. Both of these studies reported a high prevalence of HCT-CI scores over 0 (82% and 71%, respectively) among recipients of myeloablative HCT.67,72 Changes in supportive care and/or differences in laboratory reporting of DLco and methods of correction for anemia92,93 might have been responsible for over-scoring of comorbidities and, hence, lack of significance of the HCT-CI in these two studies.
Reliability studies are in progress. The CCI has good inter-rater (0.74–0.945) and test-retest reliability (0.92) rates by interclass correlation coefficient. The HCT-CI is likely to have at least the same reliability rates, given that it was a step improvement over the CCI by adding objective laboratory tests. In general, appropriate sample size and number of events are of vital importance not only for accurate validation studies but also to ensure strong inter-rater reliability. Good sample size is a known approach for reducing error of measurement compared with actual group differences since, statistically, the increase of the true variance, with increase in sample size, would triple that of the error variance, resulting in more reliable data reporting. It still remains to be tested whether data managers, medical students, or nurses could be reliable for comorbidity coding. In general, one validates not a measurement instrument, but rather some use for which the instrument was developed. The HCT-CI increasing scores were meant to be associated with a trend of worsening outcomes with different ranges based on conditioning intensity, disease risk, and other factors. Therefore, care must be used when categorizing risk groups across different populations.94
Nevertheless, overall results published so far support the validity of the HCT-CI as a reproducible and sensitive tool to capture relevant comorbidities and a powerful predictor of HCT outcomes.
Could the HCT-CI Be Augmented? Is There Any Additional Role of Inflammatory Markers?
CRP is an inflammatory biomarker thought to aid in host defense or to represent an imbalanced immune function of the host. Some results have indicated the prognostic value of pre-HCT CRP levels in predicting NRM and overall survival.90,95 One study found CRP levels to add additional prognostic information to the HCT-CI.46 Further studies are needed to clarify whether CRP levels are measurements of aggressive disease status, ongoing inflammatory/infectious process, or impaired host immunity. Whether biomarkers add additional prognostic information to comorbidities and performance status is unknown. Therefore, accounting for comorbidities and performance status in studies investigating the impacts of biomarkers is essential to ensuring accurate results and avoiding confounders.
FHCRC investigators have recently found high serum ferritin, low serum albumin, and low platelet count before HCT to have independent prognostic impacts on HCT outcomes that were additional to those of the HCT-CI.96 Serum ferritin could be another inflammatory biomarker or a representative of iron overload, which is known to increase post-HCT morbidity and mortality.97 Low serum albumin is a predictive for cardiovascular complications and mortality in the general and geriatric populations98,99 and has been used in other prognostic systems,100 but was never previously assessed as a pre-HCT predictor of outcomes. Low platelet counts might be a sensitive measure of the impacts of prior treatment on marrow function. Each of these laboratory values was assigned a score of 1 by the FHCRC investigators and incorporated into the HCT-CI. Validation of the prognostic power of the augmented HCT-CI remains to be investigated in an independent patient cohort.
Conclusions and Future Recommendations
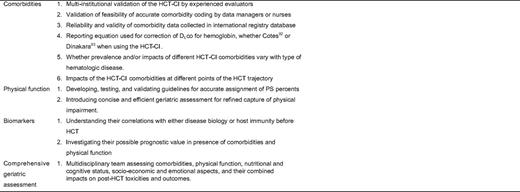
Comorbidities are now in the forefront for optimizing risk assessment before autologous or allo-HCT for hematologic diseases. The HCT-CI has been shown to sensitively capture organ comorbidities and to provide valuable prognostic information for assignments of patients to clinical trials. Multiple research questions remain to be addressed (Table 2). Good sample size, roughly equal stratification of patients into risk groups, and the inclusion of possible confounders in the analyses would support the reliable use of the HCT-CI in future studies. Aging might be more accurately thought of as a three-dimensional variable incorporating chronologic age, comorbidities, and physical function. Comprehensive geriatric assessment incorporating comorbidities, physical function, nutritional and cognitive status, and socio-economic and emotional aspects might prove of value in preventing excessive mortality and disability similar to other oncologic settings.101 We are only beginning to learn about the interplay between different comorbidities and between comorbidities and other risk factors to shape patients' profile of morbidity and mortality after HCT. Further understanding of patient variables would allow us to minimize the risks of conventional and NMA/RIC HCT through refined decision making, and possibly to develop novel preventive measures for HCT toxicities. The ultimate goal is broader applicability of this unique potentially curative treatment option with lessened mortality and improved quality of life.
Acknowledgments
The author would like to thank Bonnie Larson, Helen Crawford, Karen Carbonneau, and Sue Carbonneau for help with manuscript preparation. The author is grateful for research funding from NIH grants P01HL036444, P01CA078902, P01CA018029, P30CA015704, and K99HL088021. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH or its subsidiary institutes and centers.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests.
Off-label drug use: No drugs are approved for preparative regimens for HCT, so all discussions about therapeutics are off-label.
Correspondence
Mohamed Sorror, MD, MSc, Fred Hutchinson Cancer Research Center, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., PO Box 19024 Seattle, WA 98109; Phone: (206) 667-2765; Fax: (206) 667-6124; e-mail: msorror@fhcrc.org