Abstract
Stroke prevention in atrial fibrillation (AF) is a rapidly expanding indication for lifelong oral anticoagulation. The vitamin K antagonists (VKAs) effectively prevent stroke, but are notoriously difficult to manage and are associated with frequent adverse events. These factors account for the widespread underuse of warfarin for patients with AF who are qualified candidates for therapy. New oral anticoagulants with different mechanisms of action are beginning to exit phase III trials and may replace the VKAs for a number of indications, especially AF. The oral direct thrombin and Xa inhibitors are furthest along in development. Dabigatran etexilate, a thrombin inhibitor, has recently shown excellent outcomes in the prevention of stroke in patients with AF. The oral Xa inhibitors are still in phase III trials for stroke prevention in AF, but results from trials for other indications look promising. These short-acting, short-duration, unmonitored drugs are not without limitations and potential adverse effects. The perceived drawbacks of the VKAs may actually be assets in the management of patients with AF, and the pros and cons of each class of drug must be taken into account as physicians consider or patients request transition to a new class of oral anticoagulants.
Stroke prevention in atrial fibrillation (AF) is the underlying indication for anticoagulation in approximately 50% of patients treated with a vitamin K antagonist (VKA). AF is a growing medical concern with a current prevalence of 3–5 million persons in the United States and projections of a prevalence reaching 12–15 million over the next 40 years.1,2 Stroke is the most feared complication of AF, and its risk of occurring can be predicted by well-established risk factors for which the CHADS2 score is the best known.3 (CHADS2: C = congestive heart failure; H = hypertension; A = older than age 75 years; D = diabetes mellitus; S2 = prior stroke or history of transient ischemic attack.) The VKA, warfarin, has been shown to reduce the risk of stroke by as much as 64%.4 Warfarin therapy is recommended for moderate- to high-risk individuals, while aspirin is recommended for low-risk individuals with AF.5 Unfortunately, warfarin requires skillful and labor-intense dose management and patient communication to achieve the best outcomes; otherwise, patients are at high risk for developing major bleeding or thrombosis.6 In fact, the VKAs are among the top two or three drugs most frequently associated with serious adverse events resulting in emergency room visits or hospitalization.7 Fear of adverse events and the complexity of dose management are important factors leading to the widespread underuse of warfarin for patients with AF who are qualified candidates for therapy.8
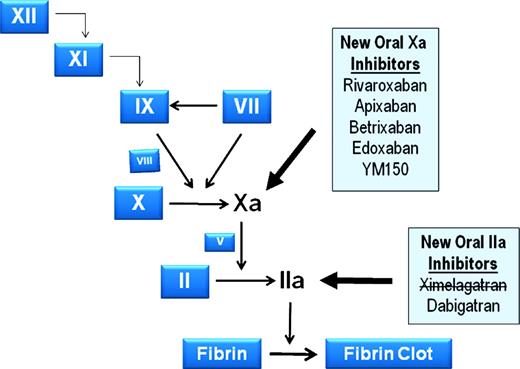
New oral anticoagulants, with distinctly different mechanisms of action, are poised to replace the VKAs and have the potential to dramatically change the way we manage patients at risk for venous and arterial thromboembolic disease. In contrast to the VKAs, which target an enzyme in the vitamin K pathway that leads to the reduction of the functional levels of factors II, VII, IX, and X, many of the new agents rely on targeting a particular coagulation factor and directly inhibiting it. Figure 1 identifies a number of new agents and their target factor. This discussion will focus on three such agents that are most advanced in development and likely to be the first to reach market in the United States.
New oral anticoagulants under development that target factor IIa and factor Xa. Participating proteins are depicted by their zymogen symbols for simplicity. Factor X and its activation to Xa, and Factor II and its activation to IIa are more fully illustrated, because these two proteases are targets of the therapeutic agents in the shaded boxes. Bold arrows from the shaded boxes point to the proteases that those agents inhibit, and an agent with a line through it has been withdrawn from development.
New oral anticoagulants under development that target factor IIa and factor Xa. Participating proteins are depicted by their zymogen symbols for simplicity. Factor X and its activation to Xa, and Factor II and its activation to IIa are more fully illustrated, because these two proteases are targets of the therapeutic agents in the shaded boxes. Bold arrows from the shaded boxes point to the proteases that those agents inhibit, and an agent with a line through it has been withdrawn from development.
The pharmacokinetic and pharmacodynamic attributes of dabigatran etexilate, rivaroxaban, and apixaban are outlined in Table 1, where they are compared to warfarin. These new drugs reach peak maximal effect within a few hours, possibly eliminating the need for a two-drug regimen to treat acute venous thromboembolism (VTE); they have predictable dose responses, thus eliminating the need for routine monitoring; and they have few if any important food or drug interactions, thus simplifying management. They do, however, have different routes of metabolism and elimination, with renal clearance playing a variable role with each drug.
Dabigatran Etexilate—A Direct Thrombin Inhibitor
Although ximelagatran was the first oral direct thrombin inhibitor to complete phase III clinical trials, including two large stroke prevention in AF trials.9,10 It has been dropped from further development because of hepatotoxicity. Dabigatran etexilate (Pradaxa, Boehringer Ingelheim, Ridgefield, CT), like ximelagatran, is an oral prodrug administered once or twice daily. It is rapidly converted by serum esterases to dabigatran, a competitive direct thrombin inhibitor. Dabigatran etexilate has a 6.5% bioavailability, a 2-hour onset of action, and a 14- to 17-hour half-life.11 Eighty percent of the drug is renally excreted, and therapy is not recommended for patients with a creatinine clearance < 30 ml/hr. Table 1 summarizes dabigatran etexilate's properties.
Dabigatran etexilate received European and Canadian regulators' approval for VTE prevention following orthopaedic surgery based on two studies that showed noninferiority to enoxaparin when the latter was given according to a European dosing regimen (40 mg daily beginning 12 hours preoperatively).12,13 Dabigatran did not achieve noninferiority, however, in the RE-MOBILIZE (DVT Prevention in Knee Arthroplasty in North American Trials)14 , where enoxaparin was dosed 30 mg BID beginning postoperatively (Table 2). Other trials comparing dabigatran etexilate to warfarin include the RE-COVER (Treatment of Acute DVT)15 trial and the RE-MEDY (Extended Treatment of DVT) (http://www.clinicaltrials.gov; identifier NCT00329238) trial for the acute and chronic treatment of VTE. The RE-COVER trial was a blinded, noninferiority trial of dabigatran etexilate, 150 mg BID, compared with INR (international normalized ratio)-adjusted warfarin (INR 2–3) for 6 months in more than 2500 patients with acute VTE, all of whom were initially treated with a 5- to 10-day course of standard parenteral heparin therapy. Recurrent VTE occurred in 2.4% of dabigatran-treated patients versus 2.1% of warfarin patients (HR [hazard ratio] 1.10; 95% CI, 0.65–1.84). Major bleeding occurred at a rate of 1.6% versus 1.9% in dabigatran and warfarin patients, respectively.
Results of dabigatran etexilate in major orthopedic surgery (Phase 3 trials)

TKR indicates total knee replacement; f/u, follow-up; THR, total hip replacement; Dab, dabigatran etexilate; qd, once daily; h, hour; post-op, postoperatively; Enox, enoxaparin; pre-op, preoperatively; bid, twice daily.
The phase II PETRO trial (Prevention of Embolic and ThROmbotic events in patients with persistent AF)16 was a double-blind, dose-escalating trial of dabigatran etexilate that tested a range of doses (50, 150, and 300 BID) and identified doses to carry forward in a large, phase III stroke prevention in AF trial (the RE-LY trial [Randomized Evaluation of Long-Term Anticoagulant Therapy, Warfarin, Compared with Dabigatran]).17
RE-LY compared dabigatran etexilate to warfarin in 18 113 patients with AF and an additional stroke risk factor. Two doses of dabigatran etexilate (110 mg twice daily and 150 mg twice daily), administered in a blinded fashion, were compared with adjusted-dose warfarin administered in an unblinded manner. The primary outcome was systemic embolism or stroke (including hemorrhagic stroke); the safety outcome was major hemorrhage defined as a reduction in the hemoglobin level of at least 2 g/dL, transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ. All outcomes were adjudicated by independent committees blinded as to treatment. Table 3 summarizes the findings. The stroke or systemic embolism rate was significantly lower with dabigatran etexilate at a dose of 150 mg twice daily (1.11%; RR [relative risk] 0.66; 95% CI, 0.53–0.82; P < .001 for superiority), compared with warfarin, and the 110 mg BID dose was noninferior (1.53%; RR 0.91; 95% CI, 0.74–1.11, P < .001 for noninferiority), compared with warfarin (1.69%). The rate of major bleeding with the 150-mg dose was not different from that with warfarin (3.11% vs 3.36%; RR 0.93; 95% CI, 0.81–1.07; P = .31), although it was significantly lower with the 110 mg dose, compared with warfarin (2.71% vs 3.36%; RR 0.80; 95% CI, 0.69–0.93; P = .003). The rates of hemorrhagic stroke with the 110- and 150-mg dabigatran etexilate doses (0.12% and 0.10%) were both significantly lower than with warfarin (0.38%), as were the rates of intracranial hemorrhage (0.23 vs 0.30 for dabigatran vs warfarin; P < .001). Other safety measures of interest are in Table 3. The study has been criticized because of the relatively high rate of major bleeding experienced in the warfarin group, compared with other large AF trials.18 The investigators attribute this to the greater use of aspirin in this study and to differences in the definition of major bleeding from other studies.18
Characteristics of study groups and outcomes for RE-LY Trial

BID indicates twice daily; D, dabigatran etexilate; RR, relative risk; CI, confidence interval; TIA, transient ischemic attack; MI, myocardial infarction; CHF, congestive heart failure; ASA, aspirin.
Outcomes of interest are highlighted in bold.
The most bothersome side effect with dabigatran etexilate was dyspepsia, which occurred significantly more commonly with dabigatran etexilate (11.8% and 11.3% in the 110- and 150-mg dabigatran groups) than with warfarin (5.8%) (P < .001 for both). Gastrointestinal adverse effects are thought to be due to dabigatran's tartaric acid core needed to create a low pH essential for the drug's absorption. Myocardial infarction also occurred more commonly with dabigatran (0.72% and 0.74% with 110 and 150 mg of dabigatran etexilate, respectively, compared with 0.53% with warfarin (P = .07 and .048, respectively). The pathophysiology for this difference is unclear, although the authors suggest that it may be due to a greater efficacy of warfarin for the prevention of myocardial infarction.18 There was no evidence of hepatotoxicity with dabigatran, as was seen with ximelagatran, during the 2-year median duration of the study.
In conclusion, dabigatran, 150 mg twice daily, was superior to warfarin in preventing stroke and noninferior to warfarin with regard to major bleeding. Dabigatran, 110 mg twice daily, was superior to warfarin with regard to major bleeding and noninferior to warfarin with regard to preventing stroke. Both doses resulted in a significant reduction of intracranial hemorrhage, compared with warfarin (Table 3), whereas they also resulted in an increase in myocardial infarctions, although this increase was only statistically significant for the 150-mg BID dose of dabigatran etexilate.
Ongoing studies are continuing to compare dabigatran etexilate to warfarin. Patients in the RE-LY trial are extending their anticoagulation (with dabigatran or warfarin) for an additional 28 months in the RELY-ABLE study (http://www.clinicaltrials.gov; identifier NCT00808067).
Rivaroxaban
Rivaroxaban (Xarelto, Ortho-McNeil [Titusville, NJ] and Bayer, Inc [Pittsburgh, PA]) is an oral direct factor Xa inhibitor administered once or twice daily. Rivaroxaban is not a prodrug. It has a 2.5- to 4-hour onset of action and 5- to 9-hour half-life.11 Rivaroxaban is metabolized partially by cytochrome P450 (CYP) 3A4 and is partially eliminated by the kidney and fecal routes. Table 1 lists rivaroxaban's properties.
Rivaroxaban was approved in Europe and Canada for VTE prevention following orthopedic surgery based on the RECORD19–22 trials, four phase III trials with differing durations of treatment, enoxaparin dosing, and orthopedic procedures (Table 4). All RECORD trials found rivaroxaban to be superior to enoxaparin in preventing VTE for both short- and long-term treatment, regardless of enoxaparin regimen with similar safety profiles. RECORD-2 (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism-2) found an excess of cardiovascular events (0.4 vs 0% with enoxaparin) after discontinuing rivaroxaban20 ; however, the low number of events prevented meaningful conclusions, and no trend was evident in pooled RECORD analysis.
Results of rivaroxaban in major orthopedic surgery (Phase 3 trials)
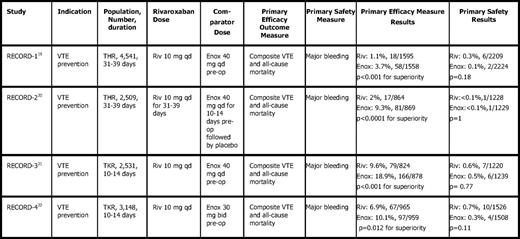
THR indicates total hip replacement; TKR, total knee replacement; Riv, rivaroxaban; qd, once daily; Enox, enoxaparin; pre-op, preoperatively; bid, twice daily.
The ROCKET AF (Rivaroxaban Once daily oral direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial is an event-driven, double-blind, noninferiority trial comparing rivaroxaban, 20 mg QD, to warfarin (INR 2–3) in more than 14,000 patients with nonvalvular AF.23 Patients must have had a history of stroke, transient ischemic or systemic embolism, or at least two additional independent risk factors for stroke to be enrolled. Criteria are in place to enrich the population with warfarin-naive patients and high-risk patients (CHADS2 score of ≥ 3). This compares with the RE-LY trial, where two-thirds of patients had a CHADS2 score of ≤ 2). Outcomes will be available in late 2010 (see http://www.clinicaltrials.gov; identifier NCT NCT00403767).
Rivaroxaban's efficacy in acute and chronic VTE treatment is being studied in the EINSTEIN trials24 (see http://www.clinicaltrials.gov; identifier NCT00440193). The Einstein Extension study24 randomized 1200 patients to rivaroxaban, 20 mg daily vs placebo, after 6–12 months of therapy for acute VTE and followed them for another 6–12 months. Recurrent VTE occurred in 1.3% versus 7.1% of rivaroxaban versus placebo patients (HR 0.18; 95% CI, 0.09–0.30; P < .0001). Major bleeding did not occur in placebo patients and occurred in 0.7% of rivaroxaban patients (P = .106). There was no fatal or critical site bleeding episodes. The results of the acute treatment trials are pending.
Apixaban
Apixaban (Bristol Myers Squibb [New York, NY]; Pfizer [New York, NY]) is an oral direct factor Xa inhibitor administered twice daily. It has a 3-hour onset and an 8- to 15-hour half-life.25 Apixaban is metabolized partially by CYP3A4, and its elimination is impaired if given with strong CYP3A4 inhibitors (eg, ketoconazole, ritonavir). Table 1 reviews apixaban's properties.
Apixaban's efficacy in VTE prevention following orthopedic surgery was compared with enoxaparin in the ADVANCE (Apixaban Dosed Orally Versus Anticoagulation With Enoxaparin) trials, including three studies with differing treatment duration and enoxaparin dosage (Table 5). ADVANCE-126 (knee arthroplasty) failed to show noninferiority of apixaban, 2.5 mg twice daily, compared with enoxaparin when the latter was dosed according to the North American regimen (30 mg BID starting ∼ 12 hours after surgery), although it was associated with significantly less overall bleeding than enoxaparin (0.7 vs 1.4%, P = .05). However, ADVANCE-2 and ADVANCE-3 (knee and hip arthroplasty)27,28 found that apixaban was superior to enoxaparin when the latter was dosed according to a European regimen (40 mg subcutaneously once daily beginning 12 hours before surgery).
Results of apixaban in major orthopedic surgery (Phase 3 trials)
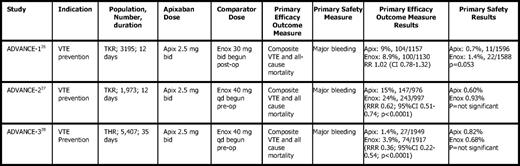
TKR indicates total knee replacement; THR, total hip replacement; Apix, apixaban; Enox, enoxaparin; bid, twice daily; Enox, enoxaparin; post-op, postoperatively; qd, once daily; pre-op, preoperatively; RR, relative risk; CI, confidence interval; RRR, relative risk ratio.
The ARISTOTLE phase III study compares apixaban, 5 mg BID, to warfarin, INR 2–3, for stroke prevention in more than 18,000 AF patients with a median CHADS2 score of 2.29 This randomized, event driven, double-blind, noninferiority study is closed to patient entry and completing follow-up. It will be reported in 2011 (http://www.clinicaltrials.gov; identifier NCT00412984).
Apixaban (2.5 mg BID) was also compared with aspirin (81–324 mg QD) for stroke prevention in AF (AVERROES trial) in more than 6000 patients who have failed or who are unsuitable for warfarin. This study was recently stopped because of the superiority of apixaban over aspirin in these patients without excess bleeding. Details of the study, however, are pending (http://www.clinicaltrials.gov; identifier NCT00496769).
Other Novel Anticoagulants for AF
Betrixaban (Portola Pharmaceuticals, South San Francisco, CA)—another oral, direct factor Xa inhibitor—has recently emerged from a phase II dose-ranging study in 508 patients with AF evaluated over a 3- to 12-month period. With a median follow-up of 147 days, the 40 mg once daily dose resulted in less bleeding than the 60- or 80-mg doses of betrixaban or warfarin at an INR 2–3.30 Phase III studies are pending. Similarly, edoxaban—an oral direct factor Xa inhibitor (Daiichi Sankyo, Inc, Parsippany, NJ)—in a phase II dose-ranging study of 1146 patients with AF, found that the once daily regimen of either 30 mg or 60 mg resulted in similar major bleeding events as warfarin, whereas the BID 30-mg and 60-mg dosing regimens resulted in higher major bleeding rates.31 Phase III studies are in progress with the Engage AF TIMI 48 trial with planned enrollment of more than 16,000 patients (http://www.clinicaltrials.gov; identifier NCT00781391). Lastly, YM 150 (Astellas Pharma, Tokyo, Japan [formerly Yamanouchi Pharmaceutical Company]), also an oral direct factor Xa inhibitor, is just entering phase III trials in AF (http://www.clinicaltrials.gov; identifier NCT00938730).
A novel change in the warfarin molecule has resulted in a new VKA, tecarfarin, which differs from warfarin with regard to pharmacokinetics, but not pharmacodynamics. It is not metabolized by the CYP 450 system, but rather by esterases, thus avoiding many of the drug–drug interactions and genetic variations found in the P450 enzymes that occur with warfarin. The expectation is that time in therapeutic range should be greater than with warfarin and that INR monitoring may need to be less intense. In a small, phase 2a study of 66 patients with AF, time in therapeutic range was improved in patients on warfarin switched to tecarfarin (59% vs 71%; P = .0009).32 In a larger mixed indication study (EMBRACE AC) of 612 patients, including patients with AF, time in therapeutic range was high in both the tecarfarin and warfarin groups (74% vs 73.2%, respectively), and the trial failed to meet its goal of superiority over warfarin as measured by time in the therapeutic range.33
The Future for Warfarin
New antithrombotic agents have many potential advantages over the VKAs, including their rapid onset of action, predictable therapeutic effect, and limited drug–drug interactions. These advantages may allow for routine therapy without monitoring and may possibly eliminate the two-drug regimen required for many thromboembolic conditions (ie, heparin followed by warfarin). All of this may result in a decrease in the burden of care for the physician, an increase in the quality of life for the patient, and greater use of anticoagulants, especially for conditions like AF, which is widely undertreated.
However, there are also potential disadvantages.34 Short half-lives of new agents make the issue of medication adherence extremely important, especially for a condition like AF, in which symptoms related to stroke are only present when stroke occurs and patients may not fully understand the importance of medication adherence. Warfarin's long, effective half-life of approximately 40 hours, may work to the providers' advantage in a nonadherent patient, Therefore, a degree of nonadherence may have a negligible effect on anticoagulation levels, compared with an anticoagulant with a short half-life.
The lack of a requirement for monitoring may also deny the physician the opportunity for patient education and the earlier detection of problems. It denies the practitioner the ability to tailor the intensity of anticoagulant therapy for patient-specific factors, such as for patients on single or dual antiplatelet therapy, or for those patients with an increased bleeding risk. Lastly, it may make it difficult to determine if the specific therapy has failed. If a patient develops a thromboembolic event on warfarin, the INR is measured to determine if the event is truly a failure of therapy or whether the patient was subtherapeutic (due to noncompliance or other factors influencing the INR). In the latter case, dosing can be adjusted to increase the INR, and patient education can be provided if thought to be necessary. With the use of a nonmonitored drug, such determinations cannot be made. Other potential disadvantages include dosing adjustment for renal and/or hepatic dysfunction. The absence of an antidote may be problematic for patients who are at a high risk of bleeding or for those who present with a bleed. This may not be as important a problem as some suggest, because rapid reversal of warfarin is not simple and requires infusions of fresh frozen plasma or factor concentrates, the latter of which have been shown to be able to reverse anticoagulation with several of the new agents.35 Specific antidotes are also being developed for factor Xa inhibitors.36
Finally, warfarin is available as a generic medication and is relatively inexpensive. New agents will be significantly more expensive and third-party payers may require prior approval based on prespecified criteria. Patients who are unable to afford their medications may sometimes not take them, or skip days to extend the supply, as recently shown in a physician survey indicating that many patients were not filling their prescriptions or were skipping their pills due to financial stresses.37
As new agents emerge from phase III studies, and if the studies have positive outcomes, the impact on warfarin prescription may be felt most in patients with AF. Patients with a condition requiring lifelong therapy with minimal to no symptoms will likely seek out such agents to improve their quality of life by eliminating the need for frequent monitoring and reducing dietary and drug–drug interaction concerns. However, warfarin will remain the mainstay of treatment for patients with mechanical heart valves, because studies in this population have not been started. Warfarin may also hold favor with patients who are considered noncompliant with therapy and as an option for those patients who “fail” or develop an event while on one of the new agents.
Disclosures
Conflict-of-interest disclosure: The author is a consultant for Daiichi Sankyo, Boehringer Ingleheim, Ortho-McNeil, Bayer, Inc, and Bristol Myers Squibb. He is also a member on Bristol Myers Squibb's Data Safety Monitoring Boards and is associated with Sanofi Aventis' Speakers Bureau.
Off-label drug use: None disclosed.
Correspondence
Jack Ansell, MD, Department of Medicine, Lenox Hill Hospital, 100 E. 77th St., New York, NY 10075; Phone: (212) 434-2142; Fax: (212) 434-2246; e-mail: jansell@lenoxhill.net