Abstract
The presence of antiphospholipid antibodies has been associated with an increased risk of recurrent pregnancy loss, and there is evidence to suggest that antithrombotic therapy improves the likelihood of a successful outcome in affected women. Recent studies suggest an association between hereditary thrombophilia and pregnancy loss, although a causal role remains controversial. Although the available data are limited and flawed, there is increasing use of antithrombotic therapy in thrombophilic women with a history of pregnancy failure. Given the absence of proven effective therapy in women with unexplained recurrent loss, there is also growing pressure to intervene with antithrombotics in women with no known underlying thrombophilia. This article reviews the evidence for an association between thrombophilia and recurrent pregnancy loss and the data regarding the use of antithrombotic therapy for prevention of loss—an area that remains particularly challenging because of the paucity of good quality data upon which to base clinical decisions.
Pregnancy loss is common, and up to 50% of human conceptions are unsuccessful.1 Most losses occur in the first trimester.1 Unfortunately, the terminology used to classify pregnancy loss is inconsistent and hampers interpretation of the literature.2 Miscarriage, which is traditionally defined as spontaneous loss before fetal viability,1 is variously used to describe loss before 24 weeks,1 20 weeks,3 or even 12 weeks.2
As many as 5% of women experience two or more early losses, and 1% to 2% have three or more early losses.1 Recurrent loss, which is defined as three or more consecutive spontaneous miscarriages with or without previous live births, is a heterogeneous condition. Known risk factors include advanced maternal age, maternal anatomic anomalies, chromosomal abnormalities, endocrine dysfunction, immunologic problems, environmental factors, and antiphospholipid antibody syndrome.1 In approximately 50% of cases,4 a cause is not identified despite extensive investigation, and affected women are at increased risk of developing clinical depression and anxiety.5
Successful pregnancy outcome is dependent on trophoblast invasion into the uterine vasculature and on the development and maintenance of an adequate uteroplacental circulatory system. It is hypothesized that inadequate placentation and damage to the spiral arteries with impaired flow and prothrombotic changes lead to placental-medicated pregnancy complications, such as fetal loss.6 The low-pressure uteroplacental system, much like the venous system, may be susceptible to thrombotic complications in hypercoagulable states.6 Animal and in vitro studies demonstrating that the hemostatic system plays an important role in placental and fetal development suggest that thrombosis is not the only mechanism for pregnancy failure in thrombophilic women. Mice trophoblast cells express both tissue factor and thrombomodulin. Structural abnormalities (thinning of the layer lining the maternal lacunae and reduced number of trophoblast cellular contacts) are seen in the placentae of tissue-factor null mice embryos,7 whereas absence of thrombomodulin is associated with mice fetal death that is not associated with fibrin deposition, suggesting a responsible mechanism other than thrombosis.8 Inactivation of the gene for protein C9 and endothelial protein C receptor gene deletion10 are also associated with mice embryo death. In vitro observations suggest that the presence of activated coagulation factors results in cell-type specific changes in trophoblast gene expression.11 Antiphospholipid antibodies (APLAs) have been shown to inhibit trophoblast proliferation12 and invasion,13 as well as embryo implantation,14 while promoting trophoblast apoptosis.15 Aspirin, unfractionated heparin (UFH), and low molecular weight heparin (LMWH) have been reported to improve trophoblast function in some in vitro studies,15,16 although other investigations suggest that heparin reduces trophoblast invasion.17
Pregnancy Loss in Women With Acquired Thrombophilia
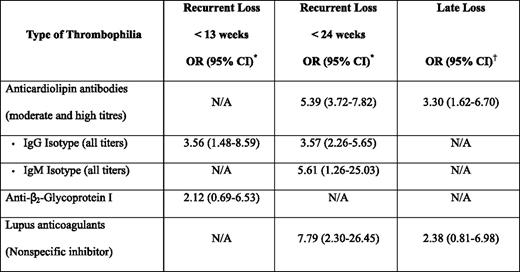
The most compelling data for a link between thrombophilia and pregnancy loss derives from studies in women with APLAs. In addition to the laboratory and animal data cited previously, there is evidence from clinical studies that the presence of persistent APLAs is associated with an increased risk of recurrent pregnancy loss.18–20 In a systematic review of 25 case control studies that sought to explore the magnitude of the risk of recurrent loss in women with APLAs, but no autoimmune disease, statistically significant associations were seen with lupus anticoagulant positivity, as well as with moderate and high titers (> 5 standard deviations above normal, > 99th percentile, or > 20 GPL/MPL [G phospholipids/M phospholipid] units) of immunoglobulin (Ig) G and IgM anticardiolipin antibodies (Table 1).19 The strongest association was seen in patients with lupus anticoagulants and with later (< 24 weeks) recurrent loss. In another systematic review, the association for nonrecurrent late (third trimester) loss was statistically significant only for elevated anticardiolipin antibodies, but not for the presence of a lupus anticoagulant.18
Pregnancy Loss in Women With Inherited Thrombophilia
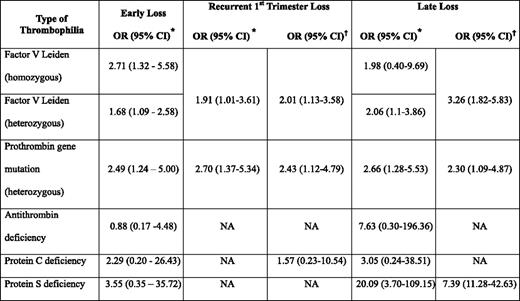
Many studies have also examined the association between hereditary thrombophilia and pregnancy loss, often with differing results,6,18,21 likely the result of heterogeneity of study design, sample size, population studied, as well as definitions of pregnancy loss and the thrombophilia studied. However, in a systematic review that examined 25 studies in 7167 women, the factor V Leiden and prothrombin gene mutations were associated with early (first or second trimester) and early recurrent loss (Table 2).18 Other meta-analyses have reported similar associations for recurrent pregnancy loss.21 Late unexplained fetal loss has also been associated with maternal hereditary thrombophilia, although results of case-control studies have again been inconsistent with some reporting an association and others identifying no association with thrombophilia. However, in one systematic review, significantly increased odds ratios (ORs) were seen for heterozygosity for the factor V Leiden mutation, heterozygosity for the prothrombin gene mutation, and for women with protein S deficiency.18 It is noteworthy that the latter positive association is based on a total of 15 patients with this thrombophilia.18
It is important to note that the magnitude of the associations observed are generally modest and that most studies included in systematic reviews and meta-analysis are case control in design and, therefore, may overestimate the magnitude of association. The NOHA (Nîmes Obstetricians and Haematologists) first study, a large case-control study nested in a cohort of nearly 32,700 women, of whom 18% had pregnancy loss with their first gestation,22 found on multivariate analysis a clear association between unexplained first pregnancy loss between 10 and 39 weeks gestation and heterozygosity for factor V Leiden (OR 3.46; 95% CI, 2.53–4.72) and prothrombin gene mutations (OR 2.60; 95% CI, 1.86–3.64), although no association was observed in losses prior to 10 weeks. However, other similarly designed studies have not documented an increased risk of pregnancy loss in carriers of the factor V Leiden23 or prothrombin gene mutation.24 Although the odds of a pregnancy loss in women with the factor V Leiden mutation was greater than in noncarriers (OR 1.52; 95% CI, 1.06–2.19) in a recently published systematic review of prospective cohort studies, the absolute risk of loss in women with this thrombophilia was small (4.2% vs 3.2% in noncarriers). There was no significant association between the prothrombin gene mutation and pregnancy loss (absolute risk of loss of 4.8% in carriers vs 3.6% in noncarriers; OR 1.13; 95% CI, 0.64–2.01).25 Thus, even those prospective studies in which thrombophilic women appeared to have an increased risk of pregnancy loss confirm that the likelihood of a successful pregnancy is high in these patients.25,26 Thus, these hypercoagulable states are likely, at most, contributing factors in the multifactorial pathogenesis of pregnancy loss.
Use of Antithrombotic Agents During Pregnancy to Prevent Loss
Antithrombotic agents typically used for prevention of pregnancy loss include (a) prophylactic doses of UFH or LMWH and/or (b) aspirin. For most indications, LMWH is the anticoagulant of choice during pregnancy. When considering antithrombotic use during pregnancy, the risks posed by any drug to the fetus must be considered, in addition to the regimen's maternal safety and efficacy.
Potential fetal complications of maternal anticoagulant therapy include teratogenicity, bleeding, and loss. UFH and LMWH do not cross the placenta27,28 and do not have the potential to cause fetal bleeding or teratogenicity, although bleeding at the uteroplacental junction is possible. Animal studies have shown that aspirin may increase the risk of congenital anomalies, but data from human studies are conflicting. Although a meta-analysis of 14 randomized studies, including a total of 12,416 women,29 reported that low-dose (50 to 150 mg/day) aspirin therapy administered during the second and third trimesters of pregnancy to women at risk for preeclampsia was safe for the mother and fetus, a review of observational studies—including more than 96,000 pregnancies—also found no evidence of teratogenicity or long-term adverse effects of aspirin during pregnancy29 ; the safety of aspirin ingestion during the first trimester remains less certain. A meta-analysis of eight studies that evaluated the risk of congenital anomalies with aspirin exposure during the first trimester found no evidence of an increase in the overall risk of congenital malformations associated with aspirin use.30 However, aspirin use during the first trimester may have been associated with a 2-fold increase in the risk for gastroschisis (OR 2.37; 95% CI, 1.44–3.88), a rare anomaly that occurs in three to six of every 100,000 births in which the intestines herniate through a congenital defect in the abdominal wall on one side of the umbilical cord.30 The reliability of this risk estimate is questionable because the use of other drugs, the type of control subjects selected, and failure to definitively confirm the diagnosis in all patients could have biased these results. One population-based observational study did note an increased risk of miscarriage with aspirin use that was greatest when aspirin was taken at the time of conception31 ; however, the number of aspirin users was small, aspirin doses were unknown, and users may have had conditions associated with an increased risk of pregnancy loss.32 Thus, there is no clear evidence of harm to the fetus with aspirin therapy and, if fetal anomalies are caused by early aspirin exposure, they are very rare.
Maternal complications of anticoagulant therapy are similar to those seen in nonpregnant patients and include bleeding (for all anticoagulants), as well as heparin-induced thrombocytopenia (HIT), heparin-associated osteoporosis, and pain at injection sites for heparin-related compounds. Information about the risk of HIT in pregnant women is limited. In one review, the risk of HIT in obstetric patients receiving prophylactic UFH for greater than 4 days was estimated at 0.1% to 1%, whereas that for obstetric patients receiving LMWH (without prior UFH) was < 0.1%.33 Long-term use of UFH has been reported to cause osteoporosis in both laboratory animals and humans. A number of studies have attempted to quantify the risk of osteoporosis during prolonged treatment (> 1 month) with UFH, and it appears that antenatal UFH is associated with clinically significant (at least 5%) bone loss.34 Results of recent investigations suggest that the use of long-term prophylactic LMWH in pregnancy is not associated with a significant decrement in bone mineral density.34 Available data suggests that the incidence of bleeding in pregnant women receiving LMWH is low. In a systematic review of 64 studies reporting 2777 pregnancies in which LMWH was used in prophylactic and treatment doses, the overall frequencies of significant bleeding were 0.43% (95% CI, 0.22–0.75%) for antepartum hemorrhage, 0.94% (95% CI, 0.61–1.37%) for postpartum hemorrhage, and 0.61% (95% CI, 0.36%–0.98%) for wound hematoma; giving an overall frequency of 1.98% (95% CI, 1.50–2.57%).35
Clinicians are increasingly using antithrombotic therapy in women at risk of recurrent pregnancy loss. Given that there is some data to show that low-risk nonpharmacologic interventions (eg, antenatal counseling and psychological support, intensive pregnancy surveillance) can result in subsequent pregnancy success rates of > 75%36,37 ; the urge to use unproven treatments that could potentially cause harm should be avoided.
Use of Antithrombotic Therapy in Unselected Women With Pregnancy Loss
Antithrombotic therapy has been increasingly prescribed in this setting based, at least in part, on the results of two trials that demonstrated higher live birth rates in women without underlying hereditary thrombophilia and at least three unexplained consecutive losses randomized to prophylactic LMWH than in those assigned to placebo or no treatment.38,39 However, both of these studies had important methodologic limitations, including a lack of blinding38 or uncertain blinding,39 relatively high rates of loss to follow-up,38,39 and, in one study, an unexpectedly low live birth rate in the placebo arm.39
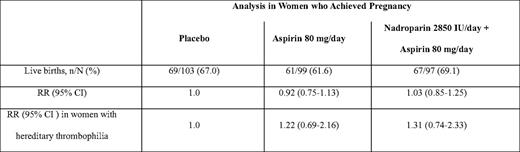
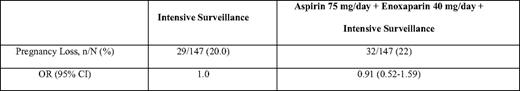
In the Anticoagulants for Living Fetuses (ALIFE) trial, 364 women with a history of two or more unexplained miscarriages at 20 weeks or less who were attempting to conceive or who had been pregnant for 6 weeks or less were randomly assigned to receive placebo, low-dose aspirin alone (80 mg), or low-dose aspirin combined with open-label prophylactic LMWH (nadroparin 2850 units subcutaneously per day).40 Women with APLAs were excluded, but a known inherited thrombophilia was not an exclusion criteria. Aspirin was commenced at randomization and continued until 36 weeks, whereas LWMH was initiated starting at 6 weeks gestation when a viable intrauterine pregnancy was confirmed. Live birth rates did not differ significantly between the three study arms (Table 3). In a prespecified subgroup analysis, no significant benefit was found in women with inherited thrombophilia, although the possibility of benefit cannot be excluded. Similar results were reported in the Scottish Pregnancy Intervention (SPIN) Study (Table 4).37 This multicenter randomized trial compared intensive pregnancy surveillance alone to a combination of intensive surveillance along with LMWH (enoxaparin 40 mg subcutaneously per day) and low-dose aspirin (75 mg per day) in 294 women with a history of two or more miscarriages at 24 weeks or earlier. Women were excluded if they were known to have APLA syndrome or another thrombophilia, or if testing at enrollment demonstrated lupus anticoagulant or IgG/IgM anticardiolipin antibody positivity.
Use of Antithrombotics in Women With APLAs
The combination of UFH and low-dose aspirin has been shown to be effective in reducing pregnancy loss in women with APLA syndrome with prior recurrent miscarriage.41 Of the interventions examined in a systematic review that summarized the data from 13 randomized or quasi-randomized trials, including a total of 849 pregnant women with APLA and a history of pregnancy loss, only UFH combined with aspirin was shown to reduce the incidence of pregnancy loss (relative risk [RR] 0.46; 95% CI, 0.29–0.71 when compared with aspirin alone); however, this conclusion is based on the results of only two studies that enrolled a total of 148 patients.41 In one trial of 50 women, the use of higher dose UFH and aspirin did not decrease the risk of pregnancy loss, compared with low-dose UFH and aspirin. On its own, aspirin (three trials, n = 71) demonstrated no statistically significant reduction in pregnancy loss, compared with usual care or placebo (RR 1.05; 95% CI, 0.66–1.68), although with the small number of total patients evaluated, a small benefit cannot be excluded.41 There was no evidence to support the use of corticosteroids.41 The combination of LMWH with aspirin had no statistically significant effect on pregnancy loss when compared with aspirin alone (RR 0.78; 95% CI, 0.39–1.57) or with intravenous γ-globulin (RR 0.37; 95% CI, 0.12–1.16); although in both cases, the point estimate was in the direction of benefit.41 Patients enrolled in the study comparing LMWH and aspirin with aspirin alone may have been at relatively low risk of recurrent loss and less likely to show benefit with aspirin, because they had low APLA titers (eg, IgG anticardiolipin antibody > 9 GPL units or IgM anticardiolipin antibody > 5 MPL units) and were enrolled into the study relatively late.42 This study has also been criticized because 24% of patients crossed over to the other treatment arm; however, the results were similar between adherent and nonadherent patient groups. A trial subsequent to this systematic review that enrolled a heterogeneous population of women with a history of two or more unexplained consecutive losses before 32 weeks gestation and either a persistent APLA, hereditary thrombophilia, or antinuclear antibody positivity, also showed no benefit to the addition of LMWH to aspirin in the APLA group (live birth rate 77.2% in those receiving LMWH and aspirin vs 75.0% in those allocated to aspirin alone); however, interpretation of these results is hampered by small sample size (n = 42). Furthermore, once again, patients with lower titer anticardiolipin antibody titers (> 15 GPL units for IgG anticardiolipin antibodies and > 25 MPL units for IgM anticardiolipin antibodies) were accepted for study participation.43 The results of randomized trials examining the efficacy of aspirin with either UFH or LMWH are detailed in Table 5.42–50
Randomized trials examining the use of aspirin with either UFH or LMWH in the prevention of recurrent miscarriage in women with antiphospholipid antibodies

aPTT, activated partial thromboplastin time; NSI, nonspecific inhibitor (lupus anticoagulant); ACA PC, anticardiolipin antibody to phosphatidyl choline; ACA PS, anticardiolipin antibody to phosphatidyl serine; ACA PI, anticardiolipin antibody to phosphatidyl inositol; ACA PG, anticardiolipin antibody to phosphatidyl glycerol; ACA PE, anticardiolipin antibody to phosphatidyl ethanolamine; IVIG, intravenous immunoglobulin.
*Control arm for RR calculation.
In a recently published meta-analysis and meta-regression of relevant randomized controlled trials, patients with APLAs and a history of recurrent loss who received aspirin in combination with either UFH or LMWH had a significantly higher live birth rate than patients who received aspirin alone (74.3% vs 58.8%, respectively; RR 1.30; 95% CI, 1.04–1.63).51 When the analysis was confined to those receiving LMWH, only a trend toward benefit was noted. No study comparing LMWH and UFH was included in either of the previous meta-analyses and the relative effectiveness of UFH versus LMWH with respect to prevention of recurrent loss in women with APLA remains unknown. The results of two recent pilot studies suggest that the combination of LMWH and aspirin might be equivalent to UFH and aspirin in preventing recurrent pregnancy loss; however, both studies have methodologic limitations and were underpowered to detect small differences.49,50 Despite the fact that there is no evidence from randomized trials that LMWH is equivalent to UFH in these patients, most centers now use LMWH in this setting because this agent is more convenient and safer than UFH.
Use of Antithrombotic Therapy in Women With Hereditary Thrombophilia
Data surrounding the use of antithrombotic therapy in women with hereditable thrombophilia and pregnancy loss are less convincing and consists of predominantly small, uncontrolled trials or observational studies with significant methodologic limitations. In one of the few randomized trials in this area, Gris and colleagues52 reported that treatment of women with a thrombophilia (factor V Leiden, prothrombin gene mutation, or protein S deficiency) and one previous pregnancy loss after 10 weeks gestation with 40-mg enoxaparin daily resulted in a significantly higher live birth rate (86%), compared with low-dose aspirin alone (29%). However, this trial has significant limitations, including small sample size, absence of an untreated control group, and inadequate concealment of allocation. Furthermore, the high rate of fetal loss in the aspirin group, compared with that seen in other studies, has led to concerns about the generalizability of this study's findings. In the LIVE-ENOX trial, in which 166 women with thrombophilia and recurrent pregnancy loss were randomized to one of two doses of enoxaparin (40 mg/day or 80 mg/day), there was no significant difference in pregnancy outcomes between the two groups; however, the rate of live birth was higher than might have been expected, given the patients' prior histories.53 There has been considerable debate about this trial focusing on its limitations, particularly the absence of an untreated control group, the heterogeneous entry criteria, and the risk of regression toward the mean with the use of a historic comparison group.
Although the data described previously provide some circumstantial evidence that LMWH may improve the pregnancy outcome in women with heritable thrombophilia and recurrent pregnancy loss or loss after 10 weeks, these studies have important methodologic limitations. The results of ongoing randomized trials are needed to prove a positive effect of antithrombotic therapy in women congenital thrombophilia and previous pregnancy loss (TIPPS: Thrombophilia in Pregnancy Prophylaxis [http://www.ClinicalTrials.gov; identifier: NCT00967382]; Effectiveness of Dalteparin Therapy as Intervention in Recurrent Pregnancy Loss [http://www.ClinicalTrials.gov; identifier: NCT00400387).
Summary and Recommendations
At present, decisions regarding the use of antithrombotic therapy in women with recurrent pregnancy loss should be made after reviewing with the patient current data and the limitations of the available studies, along with potential benefits, harms, and costs of any intervention. Although prophylactic LMWH and UFH appear safe, there are important drawbacks to their use in pregnancy, including the costs, inconvenience, and discomfort of daily injections, as well as risks of bleeding, skin reactions, and thrombocytopenia.35 Furthermore, the use of LMWH in pregnancy often results in induction of delivery and/or withholding of epidural analgesia. It is not surprising that, in this situation, women considering the use of antithrombotic therapy often give a higher priority to any potential positive impact of any treatment on the health of their unborn baby than to the avoidance of the pain, cost, and inconvenience of therapy.
Women with recurrent pregnancy losses should be screened for the presence of APLAs.54 There is general agreement among expert panels that women with recurrent loss and persistent APLA positivity should receive antepartum prophylaxis with UFH or LMWH in combination with aspirin.54–56 At present, there is good evidence that antithrombotic therapy should not be advocated for unexplained recurrent miscarriage in women without an underlying thrombophilia,37,40 but available studies do not exclude such an effect in women with heritable thrombophilia. The British Committee for Standards in Haematology has recently recommended against the antithrombotic therapy in pregnant women with a history of loss based on the results of testing for inherited thrombophilia.57 The 2008 version of the American College of Chest Physicians Clinical Practice Guidelines deleted previous weak recommendations for screening for hereditary thrombophilia in women with recurrent loss and for utilizing antithrombotic prophylaxis in women with hereditary thrombophilia and recurrent loss54 because of uncertainties associated with the magnitude of risk, potential benefits of prophylaxis, and the effect on well-being in women diagnosed with congenital thrombophilia. Ideally, these patients would be enrolled in ongoing studies. If this is not an option, affected women should be counseled that, although inherited thrombophilia may result in an increased likelihood of recurrent pregnancy loss, this is not certain and that there are no definitive data that intervention with antithrombotic therapy increases the likelihood of subsequent pregnancy success, and a decision should be made that takes patient and physician preferences into account.
Disclosures
Conflict-of-interest disclosure: The author receives honoraria from Pfizer Canada, Sanofi-Aventis Canada, and Leo Pharma Inc.
Correspondence
Shannon M. Bates, MDCM, MSc, FRCP(C), Associate Professor, Department of Medicine, McMaster University Medical Centre, 1200 Main Street West, HSC 3W11, Hamilton, Ontario, L8N 3Z5 Canada; Phone: (905) 521-2100, ext. 73928; Fax: (905) 521-4997; e-mail: batesm@mcmaster.ca
Off-label drug use: The use of LMWH to prevent recurrent pregnancy loss is an off-label indication.