Abstract
Malaria is a major world health problem. It results from infection of parasites belonging to the genus Plasmodium. Plasmodium falciparum and Plasmodium vivax cause the major human malarias, with P falciparum being the more virulent. During their blood stages of infection, both P falciparum and P vivax induce anemia. Severe malarial anemia caused by P falciparum is responsible for approximately a third of the deaths associated with disease. Malarial anemia appears to be multi-factorial. It involves increased removal of circulating erythrocytes as well as decreased production of erythrocytes in the bone marrow. The molecular mechanisms underlying malarial anemia are largely unknown. Over the last five years, malaria parasite ligands have been investigated for their remodeling of erythrocytes and possible roles in destruction of mature erythrocytes. Polymorphisms in cytokines have been associated with susceptibility to severe malarial anemia: these cytokines and malaria “toxins” likely function by perturbing erythropoiesis. Finally a number of co-infections increase susceptibility to malarial anemia, likely because they exacerbate inflammation caused by malaria. Because of the complexities involved, the study of severe malarial anemia may need a “systems approach” to yield comprehensive understanding of defects in both erythropoiesis and immunity associated with disease. New and emerging tools such as (i) mathematical modeling of the dynamics of host control of malarial infection, (ii) ex vivo perfusion of human spleen to measure both infected and uninfected erythrocyte retention, and (iii) in vitro development of erythroid progenitors to dissect responsiveness to cytokine imbalance or malaria toxins, may be especially useful to develop integrated mechanistic insights and therapies to control this major and fatal disease pathology.
Plasmodium falciparum is a protozoan parasite that causes the most virulent form of human malaria and kills at least one million children annually. The asexual blood stage parasite infects the mature red blood cell, and these stages of infection are responsible for all of the symptoms and pathologies associated with malaria. Uncomplicated malaria is linked to cyclical fevers and chills whose periodicity reflects the intraerythrocytic cycle. Severe malaria includes multiple additional pathologies including lactic acidosis, cerebral malaria (resulting from adhesion of infected erythrocytes with the endothelium in the brain) and severe anemia.1,2 Of these pathologies, severe anemia (defined as hemoglobin concentration of < 5 g/dL) remains the least understood, although it is a major health problem in endemic areas for children and pregnant women and a main cause of the infant mortality associated with malaria.3 In addition to destruction of infected and uninfected erythrocytes, inadequate response to anemia also plays a role. Plasmodium vivax, which is largely a non-lethal malaria, also causes anemia that may be severe. There are few preventative strategies. Severe malarial anemia may be managed by blood transfusion, but this carries risk of HIV and other blood-borne infections in endemic areas, as reviewed previously.3
Erythroid Interactions and Parasite Ligands
Parasite entry into erythrocytes is key to the establishment of blood stage infection and thus is central to both acute and severe malaria. Entry is a complex, dynamic process.4,5 The invading merozoite stage must orient such that its apical end containing specialized secretory organelles (called the micronemes, rhoptries and dense granules) is pointed at the erythrocyte (Figure 1A ). Interaction of the merozoite with the erythrocyte leads to the formation of a parasite-host cell junction. Invagination of the erythrocyte bilayer then results in engulfment of the parasite and establishment of the intracellular “ring-stage” parasite surrounded by a vacuolar membrane.6 There is cumulative evidence that these multiple steps of entry are mediated by parasite proteins that reside on the surface of the merozoite, its apical organelles and adhere to erythrocytes.7–9 Several hundred parasite proteins bearing a “host-targeting” (HT) or plasmodial export element (PEXEL) are also predicted to be exported to the erythrocyte during the terminal steps of invasion (or early vacuole formation) as well as intracellular parasite proliferation.10,11 Thus in principle the parasite produces a vast array of ligands that could potentially interact with targets on the host erythrocyte. A small subset of these has even been shown to adhere to membranes of uninfected erythrocytes after their release into the plasma. While it easy to understand how infected red cells with their reduced deformability and altered surface characteristics can be rapidly sequestered by the spleen leading to anemia, the central (as yet largely unanswered) question is how and which parasite ligands might influence uninfected erythrocyte clearance and their contribution to anemia.
To consider these possibilities requires a more detailed discussion of the basic biology of erythrocytic infection. Due to its complexity, invasion is an inefficient process and may be completed in only a small fraction of erythrocytes targeted for infection. Parasite antigens are also shed during entry, and thus many of these parasite-encoded erythrocyte-adhesive proteins are also present at high levels in plasma. It is possible that they may adhere to uninfected erythrocytes and this could result in IgG or complement binding to erythrocytes, leading to their clearance from circulation12–14: the enhanced destruction of uninfected erythrocytes is a prominent and perplexing feature of malarial anemia.3 In contrast, HT/PEXEL containing proteins are most likely to be released into plasma at the end of the intraerythrocytic cycle. These released proteins (especially at high concentrations) may aberrantly adhere to uninfected erythrocytes and thus trigger a signal for their removal from circulation.
Recent studies have implicated parasite proteins in rhoptries and the merozoite surface as candidates to be evaluated for their role in anemia. As indicated earlier, rhoptries are specialized secretory structures at the apical end of the invading merozoite and they release their contents at the merozoite/erythrocyte junction of invasion. The P falciparum protein RSP2 or RAP-2 has been detected on the surface of ring-infected erythrocyte membrane as well as in uninfected erythrocytes.15 Its transfer to apparently uninfected erythrocytes is thought to be due to aborted entry (see Figure 1A ) rather than adhesion of protein shed into plasma. Opsonization of RSP2/RAP-2 by specific antibodies accelerates both complement-mediated lysis as well as macrophage uptake of the targeted uninfected cells. These and data on other parasite ligands such as merozoite surface protein 7 (msp7)16 support the paradigm that parasite ligands may be presented on uninfected erythrocytes as a consequence of aborted infection.
A major limitation for rigorous testing of mechanisms is the paucity of models for malarial anemia. Evans et al17 developed a murine model of P berghei infection where severe malarial anemia (SMA) was due to accelerated loss of uninfected erythrocytes with little or no contribution from dyserythropoiesis. A strength of the model is that it mimics human malaria anemia where low parasitemias are seen concomitant with severe anemia through the duration of infection. The disadvantage is that the time needed to generate semi-immune mice requires 5 or more months with constant injections of parasites and drug combinations. A surprising finding from this model is that erythrocyte clearance may not be due to functional modifications but immunologic mechanisms. Hence, if erythrocyte clearance is induced by parasite ligands, the ligands should be evaluated for their inflammatory potential, especially invasion ligands that are currently being evaluated as vaccine candidates. Work with this model also suggests that host genetic background influences the extent of severe malarial anemia and auto-antibodies developed against uninfected erythrocytes may induce cell clearance.18 However, antibody reactivities were measured with erythrocyte ghosts, calling to question whether this is a relevant mechanism for clearance in vivo. Finally, a major advantage of this model is that P berghei is amenable to genetic manipulation, suggesting that the effect of individual ligands may be evaluated. Murine models are also limited in testing P falciparum or P vivax specific antigens, but may be useful for ligands such as MSP7 that are conserved through the genus and yet can be knocked out.
In addition to adhesive/interacting ligands, erythrocyte clearance may be linked to other changes such as oxidative damage,19 reduced deformability,20 and/or phosphatidylserine (PS) externalization.21 This is consistent with observations that in an infection, non-parasitized erythrocytes do not show gross morphological changes under light microscopy, suggesting that subtle alterations in the membrane of the erythrocyte may be responsible for their clearance.3 Thus erythrocyte signaling pathways may also play a role. Recent studies show that β2-AR–induced cAMP production results in increasing adhesiveness of normal erythrocytes,22 although adhesiveness is particularly elevated in sickle erythrocytes. Due to its membrane effects, Gαs signaling that influences malaria infection and Gαs polymorphisms linked to severe malaria23–25 could (i) underlie cellular phenotypes linked with clearance of uninfected erythrocytes in malarial anemia and (ii) be modulated by parasite ligands that adhere to erythrocytes.
Ineffective Erythropoiesis, Inflammation and Malarial Anemia
In addition to removal of infected and uninfected erythrocytes, decreased erythrocyte production and/or suppression of the erythropoietic response cause SMA. Erythropoiesis is the process by which erythroid progenitors proliferate and differentiate into non-nucleated reticulocytes in the bone marrow. It is important to note that all of erythropoiesis occurs in the context of the specialized niches termed “erythroblastic islands,” composed of erythroblasts surrounding a central macrophage in which cells proliferate, differentiate and enucleate.26 The earliest morphologically recognizable erythroblast is the proerythroblast, which undergoes 3 to 4 mitoses to produce reticulocytes. Morphologically distinct populations of erythroblasts are produced by each successive mitosis, beginning with proerythroblasts and followed by basophilic, polychromatic and orthochromatic erythroblasts. Finally, orthochromatic erythroblasts expel their nuclei to generate reticulocytes. This ordered differentiation process is accompanied by decreases in cell size, enhanced chromatin condensation, progressive hemoglobinization and marked changes in membrane organization. The intimate interaction between erythroblasts and macrophages during production of erythrocytes accounts for the critical role played by various cytokines in regulating erythropoiesis.
Unexpectedly, deficient erythropoietin production does not appear to be the cause of inadequate erythropoiesis in malaria.27 Rather, emerging evidence suggests the involvement of cytokines and other mediators of inflammation (such as hemozoin, which is parasite-derived polymerized heme). This is not entirely unexpected since cytokines are known to play a role in the maturation of erythroid of cells (reviewed in Chasis and Mohandas26). IL6 induces hepcidin expression, which is a master regulator of iron trafficking, leading to decreased iron availability for erythropoiesis; TGF inhibits erythroblast proliferation; TNFα induces cleavage of major erythroid transcription factor, GATA-1; interferon (IFN)-γ induces macrophage production of TRAIL (TNF-related apoptosis-inducing ligand). TRAIL inhibits erythroblast differentiation.
Genome-wide association studies (GWAS) have linked numerous Th1 cytokines to severe malarial anemia. Studies by Perkins and coworkers in pediatric populations suggest that decreased levels of IL12 are associated with SMA. Suppression of IL12 decreases production of IFN-γ and IFN-α; suppression of IL12 appears to be a consequence of the induction of IL10, which in turn is stimulated by infection.28,29 Children with SMA also show increased circulating levels of TNF, IL6, IL1b, IL1RA, MIP1a, MIP1b. Surprisingly, PGE and NO are suppressed (since these are usually induced by TNF), although PGE suppression may allow over-production of TNF, which is associated with enhanced severity of anemia. Reduced NO in children with SMA may promote ineffective parasite killing and in the bone marrow may suppress erythropoiesis. Circulating MIF levels may also be increased in SMA especially in murine models. But studies in human populations suggest that suppressed MIF as well as suppressed RANTES and SCGF is associated with SMA.30,31 Although the cumulative data strongly support that there is imbalance of cytokines in malarial anemia, unfortunately, there is still no direct, conclusive evidence supporting the role of these cytokines in suppressed erythropoiesis. Further, while historically, the involvement of pro-inflammatory (Th1) cytokines has been examined, recent studies suggest that Th2 cytokines such as IL4 play an important role in erythropoietic suppression.
The data on the role of Th2 cytokines on erythropoiesis come from studies in the murine malaria parasite P. chabaudi AS.32 It causes infection in mice, with blood parasitemias in excess of 20% with acute anemia (as seen in some, but not all, cases of human malarial anemia). Prior work demonstrated that IL4 interacts with the IL4 receptor (IL4R) to activate signaling through STAT6 and STAT6-/-mouse knock outs and showed increased numbers of myeloid progenitors in bone marrow and spleen. Further IL4 (and IL13 which also uses IL4R) increases iron uptake and storage in activated macrophages, and thus deviates iron to suppress the development of hemoglobin producing erythroblasts. Remarkably, STAT6 -/- mice when infected with P chabaudi displayed enhanced reticulocytosis and enhanced erythropoietin stimulation of splenocytes but decreased IFN-γ (despite higher parasitemia and the same course of anemia). Similar phenotypes were seen in wild type mice that had IL4 removed, suggesting erythropoietic suppression may occur by a STAT6-dependent mechanism. Importantly, this erythropoietic suppression can occur during acute blood stage malaria infection, suggesting that naïve infection may share common mechanistic features with severe malarial anemia in humans. The work underscores the importance of mouse models of infection in elucidating new and unexpected mechanisms important in erythropoiesis during malarial infection.
The Complexity of Nutrition and Co-infections
In regions where malaria is endemic, malaria is commonly considered to be a principal cause of severe anemia, which in turn is a major cause of morbidity and mortality. A recent, comprehensive study in Malawian children showed that along with malaria, bacteremia, hookworm and HIV were infections associated with anemia.33 Genetic disorders and nutritional deficiencies were also associated, suggesting that there clearly were multiple causes of anemia. Further, P falciparum parasitemia was strongly associated with severe anemia in areas of seasonal transmission but not in holoendemic areas, presumably because the cumulative effect of malaria is difficult to assess, as a consequence of multiple infections. Thus even in the presence of malaria parasites, additional/alternative causes of severe anemia should be considered. Malaria can modify associations between nutritional deficiency and severe anemia.33,34 Malaria is not yet reported to modify associations between genetic polymorphisms and severe anemia (although hemoglobinopathies, which manifest anemia, limit malaria parasite infection of blood cells).
Multiple reports in both murine models and human populations suggest co-infections with worms or bacteria increase associations with severe malarial anemia.35,36 This is likely because co-infections modulate the cytokine balance. Helminthic infections have been reported to make the immune response to malaria more inflammatory. For instance, in a recent study from Mali, filarial infections were found to modulate the P falciparum–specific IL-12p70/IFN-γ secretion pathways (which are IL10 dependent and thought to play a key role in resistance to malaria).36 Case fatality rates of severe malaria rise when bacteremia is present. Both gram-positive and gram-negative bacteria are seen and here again it is likely that co-infection makes the immune response to malaria more inflammatory.
Emerging Tools
The underlying causes of severe malarial anemia are multifactorial and are expected to involve both direct and indirect destruction of parasitized and non-parasitized erythrocytes, ineffective erythropoiesis, and dyserythropoiesis. In addition to animal models and GWAS studies, multiple new tools are now being brought to bear to dissect and understand the complexity of malarial anemia.
Modeling Dynamics of Immune and Erythropoietic Response in Malaria Infections
Recent computational studies have developed a mathematical model to understand how P falciparum and P vivax induce a complex immune response and severe anemia.37 In the model, immune responses incorporate a rapid response (comprising the innate component) and a slower responding (but longer term) antibody component. Several parasite stages are taken as targets for each type of immune response. A Beowulf cluster was used to simulate 8.4 × 104 combinations of parasite species, host immune response and erythropoietic response to infection. The output suggests that an ineffective innate immune response, even if antibodies are present, results in higher parasitemias.
Dyserythropoiesis reduces parasitemia slightly but aggravates anemia. Compensatory erythropoiesis reduces anemia but enhances parasitemia in the presence of an ineffective innate response. Finally sharp transitions between parasite stages of development reduce parasitemia and anemia. This model lends itself to systematic assessment of the contribution of factors in erythropoiesis and/or the innate immune response, for their cumulative effects on SMA, and suggests that treatments for malarial anemia must restore both host processes. It also suggests that P falciparum is more successful at immune evasion than P vivax.
Ex-vivo Perfusion of Human Spleen to Understand Physiology of the Human Spleen in Malaria Anemia
Splenomegaly is a common clinical manifestation of P falciparum malaria. Splenectomy predisposes to fever, higher parasitemia (with circulating mature forms of parasite) and reactivation of latent infections. A recent study suggests that SMA (rather than cerebral malaria, another fatal disease pathology) displays significant association with enlarged spleens, longer fever duration, and lower parasitemia.38 These findings have led to the hypothesis that SMA is linked to a spleen-dependent mechanism that is absent or minimally involved in cerebral malaria.
An ex-vivo perfusion system enables unprecedented access to studying directly study interactions between human spleen and human malarial infections.39 Studies using this system as well as clinical data reveal shows retention of erythrocytes infected with young parasite stages (rings). Reduced deformability of rings is well established but thought to be mild to result in retention. This retention is probably caused through an innate mechanical process while traveling through the red pulp, suggesting that minor modifications on uninfected erythrocyte surface may also underlie filtration and elimination by the spleen. Whether this is influenced by P falciparum invasion or secretome ligands targeted to erythrocytes can be comprehensively tested in this ex vivo system. The resulting data should address whether splenic retention of rings and uninfected erythrocytes may increase the risk of SMA (and thus simultaneously reduce the risk of cerebral malaria, a complication associated with high parasite loads).
In Vitro Development of Erythroid Progenitors to Dissect Responsiveness to Cytokine Imbalance or Malaria Toxins
The last 5 years have seen a steady increase in the utilization of CD34+ early hematopoietic progenitors to study commitment to and differentiation through the erythroid lineage.40 The entire process of erythroid maturation through basophilic, polychromatic, orthochromatic and reticulocyte stages to mature erythrocytes is completed in ~15 days. Flow cytometry and appropriate markers can be used to ensure that cell populations are essentially (95%) pure at an early/mid time point such as day 10 (when the cells are basophilic and polychromatic erythroblasts). Typically, ~70% to 80% synchrony is sustained through day 15, subsequent to which enucleation occurs, resulting in reticulocyte production. Both P falciparum and P vivax infect erythroid progenitors,41,42 suggesting parasitization of these cells could directly influence erythropoietin. Further monocytes/macrophages when exposed to parasites or their toxic metabolites may gender an innate immune response that stimulates ineffective erythropoiesis measurable in the in vitro culture system. Finally, since erythroid progenitors are nucleated, they are amenable to genetic manipulation. Thus, mutants in both parasite ligands and host targets may be assessed in mechanistic analyses of human erythroid cell–malaria parasite interactions underlying ineffective or dyserythropoiesis associated with severe malarial anemia.
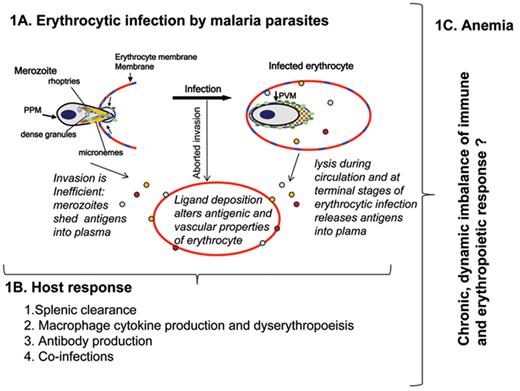
Malarial anemia: a model for the role of parasite ligands and dynamics of the host immune and erythropoietic response. A. A schematic of blood stage parasite infection. Extracellular merozoites contain specialized organelles called the rhoptries, micronemes and dense granules. Parasite proteins (blue orange and red dots) from these organelles are delivered to the junction of invasion (that concentrates rafts shown in blue) between the merozoite and the erythrocyte. Invasion is a rapid, highly inefficient process and thus may be aborted but nonetheless result in antigen deposition on the erythrocyte. Parasites that become intracellular continue to secrete proteins to remodel the erythrocyte as they mature (for 48 hours for Plasmodium falciparum). When infected erythrocytes rupture, parasite proteins are released in plasma. Parasite protein release in plasma also occurs when merozoites fail to invade erythrocytes. A subset of these are erythrocyte adhesive and may deposit on uninfected erythrocytes to change their antigenic and vascular properties. B. 1). Uninfected and infected erythrocytes remodelled by parasite as shown in panel A may be filtered by the spleen. This could be due to mechanical filtration as well as an inflammatory response. 2). Nurse cell macrophages secrete cytokines that are critical to maturation of erythrocytes. Stimulation of Th1 as well as (unexpectedly) Th2 cytokines like IL4, by multiple components shown in panel A, may promote diserythropoiesis. 3 and 4). Antibody production and coinfections could modulate both splenic and macrophage functions and thus exacerbate anemia. C. Initial modelling studies suggest that events triggered in A create imbalance in both the immune and hematopoietic responses shown in B, such that both must be restored to overcome this complex disease pathology.
Malarial anemia: a model for the role of parasite ligands and dynamics of the host immune and erythropoietic response. A. A schematic of blood stage parasite infection. Extracellular merozoites contain specialized organelles called the rhoptries, micronemes and dense granules. Parasite proteins (blue orange and red dots) from these organelles are delivered to the junction of invasion (that concentrates rafts shown in blue) between the merozoite and the erythrocyte. Invasion is a rapid, highly inefficient process and thus may be aborted but nonetheless result in antigen deposition on the erythrocyte. Parasites that become intracellular continue to secrete proteins to remodel the erythrocyte as they mature (for 48 hours for Plasmodium falciparum). When infected erythrocytes rupture, parasite proteins are released in plasma. Parasite protein release in plasma also occurs when merozoites fail to invade erythrocytes. A subset of these are erythrocyte adhesive and may deposit on uninfected erythrocytes to change their antigenic and vascular properties. B. 1). Uninfected and infected erythrocytes remodelled by parasite as shown in panel A may be filtered by the spleen. This could be due to mechanical filtration as well as an inflammatory response. 2). Nurse cell macrophages secrete cytokines that are critical to maturation of erythrocytes. Stimulation of Th1 as well as (unexpectedly) Th2 cytokines like IL4, by multiple components shown in panel A, may promote diserythropoiesis. 3 and 4). Antibody production and coinfections could modulate both splenic and macrophage functions and thus exacerbate anemia. C. Initial modelling studies suggest that events triggered in A create imbalance in both the immune and hematopoietic responses shown in B, such that both must be restored to overcome this complex disease pathology.
Disclosures Conflict-of-interest: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Acknowledgments
Work from our laboratories were supported by grants to KH (NIH: R01 AI 039071, R01 HL 079397, R01 HL069630, P01 HL 078826) and to NM (P01 HL 078826).
References
Author notes
Center for Rare and Neglected Diseases, University of Notre Dame, South Bend, IN
Red Cell Physiology Laboratory, New York Blood Center, New York, NY