Abstract
Allogeneic stem cell transplantation (allo HCT) is a curative treatment for follicular lymphoma, but is hampered by a relatively high treatment-related mortality and by difficulties in identifying high-risk groups for whom transplant is warranted. Results with myeloablative conditioning have improved, but the field has shifted largely to reduced-intensity conditioning and non-myeloablative transplantation, though morbidity and mortality are also substantial. Some groups have investigated T cell–depleted transplantation, which results in a low rate of chronic graft-versus-host disease (GVHD) and, in most studies, excellent rates of disease control. Overall, outcome after alloHCT for follicular lymphoma correlates more with disease status, with performance status and with comorbidities than with any particular conditioning regimen used. For patients with chemotherapy-sensitive disease, the treatment-related mortality has stabilized in the 15% to 20% range and, depending on the method of GVHD prophylaxis and the donor type, there is an additional 20% to 60% incidence of chronic GVHD. For patients with chemotherapy-refractory disease, both treatment-related mortality and recurrence rates are much higher, but their prognosis is dismal with other treatments and some may be cured, particularly with myeloablative transplants. Ongoing studies focus on improving conditioning regimens, on prevention of disease recurrence and on decreasing chronic GVHD.
Myeloablative Stem Cell Transplantation
Allogeneic transplantation was initially pioneered as a treatment for acute and chronic leukemias and data on indolent lymphoma patients were scarce. The durable remissions obtained with autologous transplantation challenged the conventional concept of incurability. Studies of minimal residual disease showed a close correlation between lymphomatous involvement of the autologous graft and disease recurrence. This provided the initial rationale for myeloablative allogeneic stem cell transplantation (alloHCT), which was pioneered in patients with extensive marrow involvement. Mainly because of historical precedent, total body irradiation (TBI)–containing regimens were used in early transplant series for follicular lymphoma. We reported a retrospective study on 10 patients with refractory indolent lymphoma who underwent sibling myeloablative alloHCT at MD Anderson Cancer Center.1 All had bulky disease and an average of 50% bone marrow involvement. Eight achieved remission and only 1 relapsed.2 This observation was confirmed by other groups,3,–5 and contrasted with results of alloHCT in aggressive lymphoma where recurrence rates and treatment-related mortality (TRM) were much higher. It was followed by an IBMTR registry analysis of 113 patients reported over a 12-year period (1984–1995) by 50 teams worldwide, an average of 2 patients per team.6 The registry analysis confirmed a very high rate of durable disease control after myeloablative alloHCT for indolent lymphoma. The cumulative incidence of disease recurrence was only 16%. TRM was high, particularly in those with refractory disease (the large majority in this series) or those with decreased performance status (more than 30% of patients). Age over 40 years was an additional predictor of adverse outcomes; interestingly, TBI, used in 75% of patients, was associated with better survival than busulfan/cyclophosphamide (BuCy) conditioning. A European registry analysis also found very similar overall survival (OS) and progression-free survival (PFS) for myeloablative alloHCT.7 In a case control analysis they found that allogeneic transplantation had less relapse than autologous transplantation, but alloHCT was inferior because of the increased TRM. The bulk of the data was therefore consistent: myeloablative alloHCT was predominantly used in younger patients with very advanced and end-stage disease and could reliably induce durable remissions, including in some patients with otherwise refractory disease. But the outcomes were heavily influenced by patient and disease status (Table 1 ).
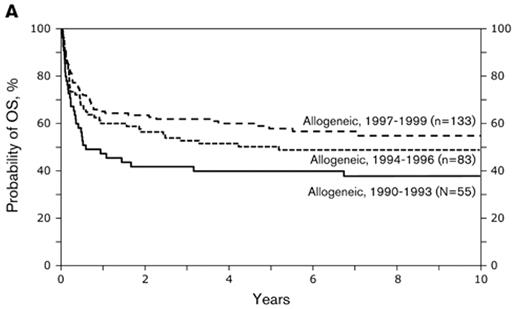
Subsequently IBMTR reported 176 patients with follicular NHL who during the period 1990 through 1999 underwent sibling myeloablative alloHCT.8 The 5-year TRM rates were 30% and recurrence rates were 21%. Of interest, outcomes improved over time: the TRM after myeloablative alloHCT declined and outcomes improved for consecutive 3-year intervals. For patients transplanted in the 1997–99 era, 2-year survival was approximately 75%. This improvement was due to two factors: Patient selection and improvements in supportive care, including advances in management and prevention of infectious complications and improvement in transfusion support. Figure 1 shows an update as of late 2008, on patients who underwent myeloablative allogeneic transplant during the 1990s. The median follow-up for survivors in this cohort is 8 years. Five-year survival for those transplanted between 1997 and 1999 is 58% (updated data: Parameswaran Hari and Jeanette Carreras, Center for International Blood and Marrow Transplant Research, Milwaukee, personal communication). The continued improvement in myeloablative transplantation is further illustrated by a recent report from the Toronto group.9 They treated 36 patients with BuCy conditioning and 1 patient with cyclophosphamide-TBI. Standard methotrexate and cyclosporine graft-versus-host disease (GVHD) prophylaxis was utilized. Median age was 43 years (range 22–58) and 33 of the patients had chemotherapy-sensitive disease. Donors were HLA-identical siblings in 27, identical twins in 4 and mismatched/unrelated in 6 cases. With a median follow-up for survivors of 65 months (1–193), 5-year OS was 79%. Six patients died from treatment-related complications and 1 from breast cancer. Only one patient relapsed. Chronic GVHD occurred in 54% of the patients.
It has long remained uncertain whether these results could be extrapolated to unrelated donor transplantation. We recently reported an NMDP analysis on 238 patients with lymphoma who underwent myeloablative unrelated donor transplant between 1991 and 2004.10 As anticipated for a series that includes patients transplanted almost 20 years ago, many had unfavorable characteristics and HLA-typing often was limited to serologic typing. Fifty-two patients had follicular lymphoma. TRM for follicular lymphoma patients was estimated at 42% at 1 year. Their 2-year PFS was also 42%
Reduced-intensity Transplantation
Giralt et al in Houston, Storb’s group in Seattle and the Hadassah group all showed around the same time that TBI-based myeloablation or high-dose busulfan were not required for engraftment of allogeneic marrow. Pre-transplant immunosuppression with low-dose radiation or fludarabine in combination with other drugs is sufficient, particularly when accompanied by further post-transplant immunosuppression.
These observations led to reduced-intensity conditioning (RIC) regimens, hypothesized to be less toxic than myeloablative conditioning, both from reduced duration of cytopenias and less extra-medullary toxicities (eg, mucositis). Because of their decreased intensity, these regimens were thought to be particularly appropriate for older patients and those with comorbidities. And several retrospective and case control comparisons indeed suggest a decreased non-relapse mortality for older patients, particularly for those with acute leukemia.11,–13 It was also thought that graft-versus-leukemia or lymphoma (GVL) effects would be able to induce durable remissions rendering intensive chemotherapy superfluous.14 Follicular lymphoma was considered an excellent target disease because of its indolent course and its susceptibility to donor lymphocyte infusions (DLI). For example, Mandigers et al reported that 2 poor-risk, relapsed low-grade NHL patients failing T cell–depleted sibling-matched allografts re-entered durable complete remission after DLI from the original donors.15 Russell et al also showed a high rate of response in indolent and mantle cell lymphoma,16 and Bloor et al showed a 76% response rate to DLI, many of them were durable.17
Non-myeloablative (NMA) and RIC regimens have proliferated and are widely used and studied. One challenge with analyzing the results is the ill defined nature of the concept. Strictly speaking, NMA conditioning regimens are those that routinely allow recovery of autologous marrow function in the absence of an allogeneic graft. RIC regimens require stem cell support but are considered less intense and toxic than the classic TBI or BuCy regimens, based on somewhat arbitrary criteria.
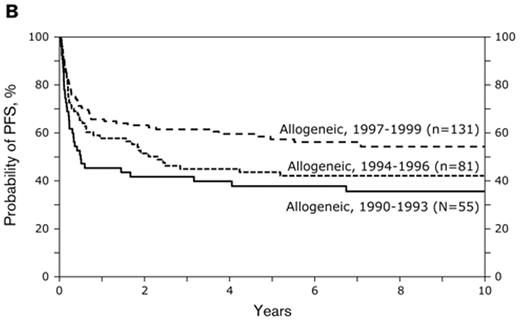
Several large transplant groups have now reported long-term outcomes of NMA or RIC at single institutions or in a cooperative group setting (Table 2 ). Four such studies are briefly summarized. The Seattle NMA conditioning regimen uses low-dose TBI with fludarabine. GVHD prophylaxis consists of cyclosporine and mycophenolate mofetil. This regimen causes limited myelosuppression and can safely be administered in the outpatient setting. They reported outcome data on 62 patients with a history of indolent lymphoma treated on a prospective study conducted at 10 centers worldwide.18 Six patients had small lymphocytic lymphoma, 2 had marginal zone lymphoma and 54 had follicular lymphoma. At the time of transplant 16 of the 54 patients with follicular lymphoma had transformed disease and 14 had grade III follicular lymphoma, leaving only about 24 patients with indolent follicular disease. Median age was 54 (range 33–66) and 45% had unrelated donors. Thirty-seven had refractory disease. Graft failure occurred in 2 individuals, grade II–IV acute GVHD in 63% and extensive chronic GVHD in 47% of patients. The cumulative incidence of TRM was 42%, with a relapse incidence of 14% for those with indolent disease (Figure 2 ). Patients with transformed lymphoma did rather poorly, as did those with unrelated or mismatched grafts. The best outcomes were seen in the 26 patients with indolent disease and related allografts; they had estimated 3-year OS and PFS rates of 67% and 54%, respectively, and a 3-year cumulative TRM of 23%. In a related report, Sorror et al from Seattle compared NMA with myeloablative alloHCT for lymphoid malignancies.19 Patients in the NMA group were older, had more previous treatment and more comorbidities and more frequently had unrelated donors. But they also more often had malignancy in remission compared with patients in the myeloablative group. Patients with comorbidities experienced lower TRM and better survival after NMA conditioning, but for patients without comorbidities, there were no differences in survival. The authors conclude that NMA is a superior procedure for patients with comorbidities. It is also possible that unrecognized imbalances in the two patient groups were responsible for the differences in outcome. For example, poor performance status is an important adverse prognostic feature, but was not included in the comparison.20 At Seattle those undergoing NMA conditioning tend to have a better performance status.21
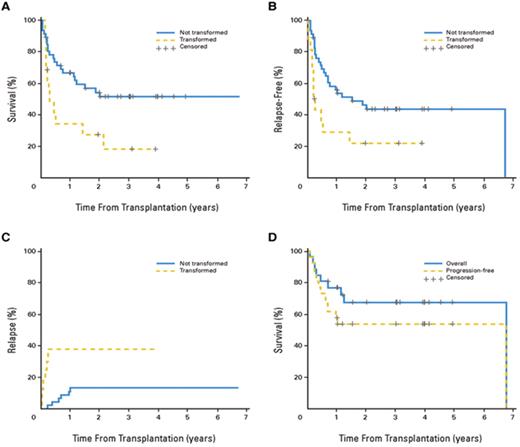
The best results of NMA/RIC were reported by Khouri et al from MD Anderson.22 They treated 47 patients with follicular lymphoma, median age 53 years (range 33–68), with a regimen consisting of fludarabine (30 mg/m2 daily for 3 days), cyclophosphamide (750 mg/m2 daily for 3 days), and rituximab (375 mg/m2 for 1 day plus 1000 mg/m2 for 3 days, on day −1, day +1 and day +8). Rituximab was included to increase response and to mitigate GVHD. Post-transplant prophylaxis consisted of tacrolimus and mini-methotrexate for 45 patients with sibling donors. The 2 patients with unrelated donors also received antithymocyte globulin (ATG). Nine patients had disease relapse after a prior autoHCT, but all had chemosensitive disease and the median IPI score at the time of transplant was 1 (range 0–3), suggesting that most had an excellent performance status in addition to excellent disease control. There was 1 case of primary graft failure and 2 cases of secondary graft failure. All 3 remain in remission, 1 after a second transplant, 1 with subsequent rituximab therapy and 1 with no further treatment. Two other patients had rapid decline in donor chimerism, which responded to DLI. With a median follow-up of 60 months (range 19–94), the estimated OS and PFS are 85% and 83%, respectively (Figure 3 ). Seven patients (15%) died of treatment-related causes, mainly viral, fungal and bacterial infections, most of them in the first year after transplant. Only 2 patients relapsed, and both responded to further treatment. Extensive chronic GVHD occurred in 19 patients for a cumulative incidence of 36% (25%–53%). These data demonstrate that an RIC regimen can be curative in patients with multiple recurrences of follicular lymphoma, who remain chemosensitive and who have an HLA-identical sibling.
As part of a larger study of RIC for lymphoma, Armand et al from the Dana Farber treated 13 patients with indolent lymphoma with a combination of fludarabine and low-dose busulfan.23 A variety of methods of GVHD prophylaxis were used. Eighty-two percent of patients in this series had chemotherapy-sensitive disease and only 43% had matched related donors. None of the patients with follicular lymphoma died of treatment-related causes, but 3-year risk for disease progression was 41% and the incidence of extensive chronic GVHD was 60%. Rodriguez et al, for City of Hope Cancer Center, reported a 21% risk for disease recurrence after a reduced intensity fludarabine-melphalan conditioning regimen for follicular lymphoma.24
Several registry studies have also been reported on reduced intensity and NMA conditioning for low-grade lymphoma. Robinson et al reported an EBMTR analysis of NMA transplantation in lymphoma.25 A variety of conditioning regimens were used. The most common ones were fludarabine-cyclophosphamide (22%), fludarabine-busulfan (16%) and fludarabine-melphalan-alemtuzumab (33%). Of the 188 patients, 55 (median age of 46 years) had low-grade lymphoma. Thirteen of them had failed autologous transplant, 44 had chemotherapy-sensitive disease and the large majority had related donors. For patients with low-grade non-Hodgkin lymphoma the year risk of TRM was 31% and the risk of disease recurrence was 21%. PFS at 2 years was 54%. In multivariate analysis refractory disease at transplant was associated with an increased risk of progression, and worse survival. Age over 50, was a risk factor for TRM.
Kusumi et al reported 45 patients, median age 45 years (32–66) with indolent lymphoma who underwent RIC in Japan.26 A variety of conditioning regimens, mostly fludarabine based and rarely incorporating TBI, were used. GVHD prophylaxis also varied, and 30 of the 45 patients had related donors. In this series with a median follow-up of 2 years, PFS was estimated at 83% for chemosensitive indolent lymphoma and 64% for chemotherapy refractory disease. Nine of 45 patients died of treatment-related complications.
The French Société Française de Greffe de Moelle reported outcomes of 73 patients with relapsed or refractory indolent lymphoma allografted after a RIC regimen between 1998 and 2005.27 The most widely used regimens were fludarabine with busulfan and ATG (n = 43) and fludarabine with TBI (n = 21). Prior to allografting, 21 patients were in complete response, 33 in partial response and 19 had chemoresistant disease. The median follow-up was 37 months (range 16 to 77 months). In patients in CR, PR and chemoresistant disease, the 3-year PFS rates were 66%, 52% and 32%, respectively. The 3-year cumulative incidences of TRM were 32%, 28% and 63%, respectively. The incidence of relapse was 9.6%.
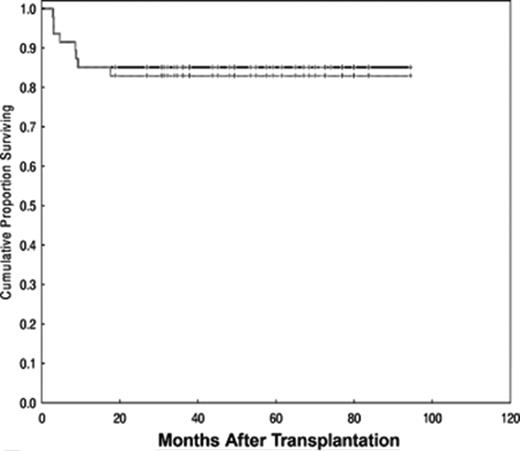
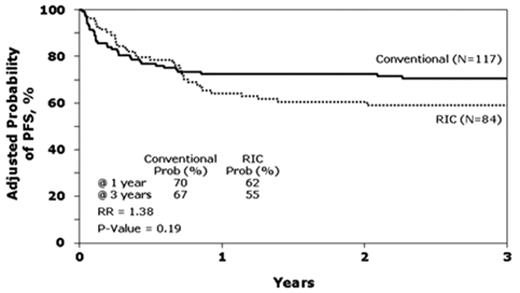
Lastly, CIBMTR compared traditional myeloablative conditioning regimens (n = 120) to RIC (n = 88) in patients with follicular lymphoma transplanted with HLA-identical siblings between 1997 and 2002.28 Consistent with the data from single institution studies, patients receiving RIC were older and had a longer interval from diagnosis to transplant. These differences did not correlate with outcomes. Median follow-up of survivors was 50 months (4–96 months) after myeloablative conditioning versus 35 months (4–82 months) after RIC (P < .001). At 3 years, OS for the myeloablative and RIC cohorts were 71 and 62 and PFS, 67% and 55%, respectively. These differences were not significant (Figure 4 ). By contrast, lower Karnofsky performance score and resistance to chemotherapy were associated with higher TRM, lower OS and PFS. In multivariate analysis, an increased risk of lymphoma progression after RIC was observed (relative risk = 2.97, P = .04). Of interest, the use of RIC regimens increased from 10% of transplants in 1997 to 80% in 2002, establishing RIC as a de facto standard in alloHSCT for follicular lymphoma.
Syngeneic Transplantation and T-cell depleted transplantation
It is clear that recurrence rates are lower after allogeneic than after autologous transplantation. Most commonly this is attributed to GVL effects but it is equally plausible that the difference in disease recurrence rates is mediated by the infusion of a tumor-free graft after alloHCT. In order to investigate the mechanisms of disease control after allogeneic transplant, Bierman and Sweetenham compared the outcomes of syngeneic with those of allogeneic and autologous transplantation.29 GVL effects are thought to be absent or minimal after syngeneic transplant. The most striking finding from this study was the low recurrence rate after syngeneic transplantation, markedly reduced compared to autologous, and similar to that after allogeneic transplant. This suggests that a tumor-free graft is an essential contributor to the success of allogeneic transplantation and that GVL effects may be less important. These observations, while limited because of low numbers, provide a powerful rationale for the exploration of T cell–depleted transplantation, particularly with the hope of reducing chronic GVHD and a number of associated late complications (Table 3 ). Indeed chronic GVHD has major effects on quality of life and on life expectancy. In a large series on survivorship after allogeneic transplantation, it was estimated that more than 20% of survivors with cGVHD were destined to die within a 10-year span, mostly from non-relapse causes.30 This risk was even higher for patients over the age of 45.
Juckett reported for the Wisconsin group on 16 patients with indolent lymphoma, median age 43 (27–62) who received conditioning with a myeloablative TBI-based conditioning regimen followed by infusion of a graft that was in vitro T-cell depleted with T10B9 antibody and post-transplant GVHD prophylaxis consisting of cyclosporin alone.31 Twelve of the 16 had matched related donors. Eleven had chemotherapy-refractory disease. With a median follow-up of 6 years, those with chemotherapy-resistant and sensitive disease had PFS of 55% (95% CI 25%–85%) and 80% (95% CI 45%–100%), respectively. Four patients died of complications and 2 with refractory disease relapsed. Two of the survivors developed extensive chronic GVHD. Similar results were reported by Mandigers et al and Verdonck et al, both from the Netherlands, who used in vitro T cell–depletion methods.32,33 These older studies provided proof of principle that durable remissions can be obtained with T cell–depleted transplants for indolent lymphoma, but the technologies used for T-cell depletion in these studies are no longer available. Recently several groups have reported results of transplant using in-vivo or in-vitro alemtuzumab for T-cell depletion.
Novitzky et al used myeloablative conditioning combined with “alemtuzumab-in-the bag.”34 They included 12 patients with follicular lymphoma. Median age was 47 (21–57). All had sibling donors. Two died of TRM and one relapsed. All others remained in remission with a median follow-up of more than 2 years. Ingram et al reported 44 patients, 28 with matched sibling donors and 16 with unrelated and/or mismatched donors. Median age was 48 (31–59) and 4 had refractory disease.35 Conditioning consisted of BEAM supplemented with alemtuzumab 10 mg to 20 mg given on days −5 to day −1. Post-transplant oral cyclosporine was given and donor lymphocyte infusions administered for falling chimerism in 7 patients. There were 10 treatment-related deaths (20%), mostly due to viral infections, a cumulative incidence of chronic GVHD of 20% and a rate of disease recurrence of approximately 20%.
Morris et al used the fludarabine melphalan regimen combined with alemtuzumab.36 They observed a TRM of only 8% and no cases of extensive chronic GVHD, but though most patients had chemotherapy-sensitive disease at time of transplant, the rate of disease recurrence was 44%. Recurrence often responded to DLIs.
Novel Approaches
Disease recurrence remains a major issue in patients with chemotherapy refractory disease. Several groups are investigating new conditioning regimens with potentially improved therapeutic ratio. Examples include the use of targeted dose busulfan or the investigational use of clofarabine in conditioning regimens. Perhaps of most interest is the incorporation or radiolabeled antibodies in NMA conditioning regimens for allogeneic transplantation.37 Targeted radiotherapy may exploit the superior anti-lymphoma activity of TBI, but avoid the side effects of TBI. The results of these trials are eagerly awaited.
There is also great interest in developing methods that prevent GVHD while maintaining GVL effects. The Stanford group has pioneered a transplant conditioning using total lymphoid irradiation and ATG.38 This results in a very low incidence of GVHD, presumably by sparing NKT cells. In a recent update they reported encouraging results including approximately 50% event-free survival in patients with lymphoid malignancies. Unfortunately few patients with follicular lymphoma participated in this trial. The Dana Farber group has pioneered the mTor inhibitor rapamycin for GVHD prophylaxis in allogeneic transplantation. mTor inhibitors have anti-lymphoma activity and, in a retrospective analysis, they found that rapamycin reduced disease recurrence in recipients of NMA transplantation.39 Lastly rituximab has some activity in treatment of GVHD, and it is increasingly being incorporated in post-transplant maintenance for prevention of recurrence.22 While rational and convenient, this is not entirely an innocuous approach. In our experience rituximab frequently causes profound neutropenia when administered after transplant and can exacerbate pre-existing immunocompromise.
DLI induces a high rate of response in patients with follicular lymphoma and has been used prophylactically to prevent disease recurrence, particularly after T cell–depleted transplantation. Unmanipulated DLI carries a risk of chronic GVHD that is proportionate to the number of T cells infused. Some groups have given DLI to patients with mixed chimerism, assuming that they are at high risk for disease recurrence. The correlation of mixed chimerism with disease recurrence is, however, not well established.23 It may depend on the particular conditioning regimen and GVHD prophylaxis method, and even on the method and cell populations used to measure chimerism.
Conclusion
Altogether the results of NMA, reduced-intensity and myeloablative transplants with or without T depletion confirm that allogeneic sibling transplant can cure patients with follicular lymphoma. The field has shifted largely to RIC and NMA transplant, though there are no convincing data that this is either a safer or more efficacious strategy for indolent lymphoma. The median age of patients in RIC series is approximately 5 years higher than in series of myeloablative alloHCT and they tend to have more comorbidities. But they are also more frequently in remission and have a better performance status than those undergoing myeloablative alloHCT. There is more conditioning-related mortality with myeloablative conditioning and there may be more GVHD-related morbidity and mortality with T-replete RIC/NMA conditioning. Overall outcome after alloHCT for follicular lymphoma correlates more with disease status, with performance status and with various measures of comorbidity than with any particular conditioning regimen used. For patients with chemotherapy-sensitive disease, the TRM has stabilized in the 15% to 20% range and, depending on the method of GVHD prophylaxis and the donor type, there is an additional 20% to 60% incidence of chronic GVHD. These are outcomes that may be unacceptable for patients with an indolent disease and a difficult to predict course, who increasingly have other life prolonging options, even when they fail autologous stem cell transplantation.40 For patients with chemotherapy-refractory disease, both TRM and recurrence rates are much higher, but prognosis is dismal with other treatments and some patients may be cured, particularly with myeloablative transplants.
Our recommendations for allogeneic transplantation are based on the pattern of recurrence, the donor type and the fitness of the patient. We recommend allogeneic transplantation for patients whose lymphoma is behaving in an aggressive fashion (ie, early recurrence after intensive front line or salvage therapy) provided they have an excellent performance status, minimal comorbidities and an HLA-identical donor (related or unrelated). But autologous transplantation is an excellent alternative for some. We routinely attempt to reinduce patients prior to transplant, but will also accept patients with refractory disease. Conditioning regimen and GVHD prophylaxis remain a matter of active investigation and ideally the patient should be enrolled on investigational protocols. For purposes of comparison of results, future studies and reports should routinely include data on performance status and comorbidities.
Myeloablative conditioning.
| Author, ref . | N . | Conditioning . | Age, y (range) . | HLA SIB/other donor . | Chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; TBI, total body irradiation; Cy, cyclophosphamide; Bu, busulfan; MEL, melphalan; NS, not stated specifically | |||||||||||
| *Four T cell–depleted transplants | |||||||||||
| Van Besien1 | 10 | TBI | 37 (23–45) | 10/0 | 0 | 72 | 70 | 80 | 20 | 0 | 50 |
| Van Besien6 | 113 | TBI 84% Non-TBI 16% | 38 (15–61) | 113/0 | 63 | 25 | 49 | 49 | 40 | 16 | – |
| Van Besien6 | 176 | TBI 68% Non-TBI 32% | 42 (22–64) | 100%/0 | 67 | 36 | 45 | 51 | 30 | 21 | 24 |
| Hari28 | 120 | TBI 75% Non-TBI 25% | 44 (22–70) | 100% | 72 | 50 | 67 | 71 | 25 | 8 | 46 |
| Stein5 | 15 | Cy/TBI 93% | 43 (31–50) | 95/5 | 95 | 60 | NS | 15 | 53 | 33 | – |
| Peniket7 | 231 | Various | 39 (19–66) | 96%/4% | 80 | 60 | 43 | 51 | 38 | 25 | – |
| Toze3 | 26 | Various* | 42 (20–52) | 20/6 | 46 | 29 | 54 | 58 | 30 | 18 | 54 |
| Forrest4 | 24 | Bu/Cy±MEL 92% | 44 (33–52) | 23/3 | 96 | 28 | 78 | 78 | 21 | 0 | 45 |
| Kuruvilla9 | 37 | BuCy | 43 (22–58) | 81%/19% | 89 | 65 | 79 | 76 | 16 | 3 | 54 |
| Author, ref . | N . | Conditioning . | Age, y (range) . | HLA SIB/other donor . | Chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; TBI, total body irradiation; Cy, cyclophosphamide; Bu, busulfan; MEL, melphalan; NS, not stated specifically | |||||||||||
| *Four T cell–depleted transplants | |||||||||||
| Van Besien1 | 10 | TBI | 37 (23–45) | 10/0 | 0 | 72 | 70 | 80 | 20 | 0 | 50 |
| Van Besien6 | 113 | TBI 84% Non-TBI 16% | 38 (15–61) | 113/0 | 63 | 25 | 49 | 49 | 40 | 16 | – |
| Van Besien6 | 176 | TBI 68% Non-TBI 32% | 42 (22–64) | 100%/0 | 67 | 36 | 45 | 51 | 30 | 21 | 24 |
| Hari28 | 120 | TBI 75% Non-TBI 25% | 44 (22–70) | 100% | 72 | 50 | 67 | 71 | 25 | 8 | 46 |
| Stein5 | 15 | Cy/TBI 93% | 43 (31–50) | 95/5 | 95 | 60 | NS | 15 | 53 | 33 | – |
| Peniket7 | 231 | Various | 39 (19–66) | 96%/4% | 80 | 60 | 43 | 51 | 38 | 25 | – |
| Toze3 | 26 | Various* | 42 (20–52) | 20/6 | 46 | 29 | 54 | 58 | 30 | 18 | 54 |
| Forrest4 | 24 | Bu/Cy±MEL 92% | 44 (33–52) | 23/3 | 96 | 28 | 78 | 78 | 21 | 0 | 45 |
| Kuruvilla9 | 37 | BuCy | 43 (22–58) | 81%/19% | 89 | 65 | 79 | 76 | 16 | 3 | 54 |
Reduced-intensity and non-myeloablative transplantation.
| Author, ref . | N . | Conditioning . | Age, y (range) . | HLA SIB/0ther donor . | Chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| *Among 62 patients were several with transformed or aggressive follicular. | |||||||||||
| **Outcome in subset of patients with indolent (mostly follicular) and HLA identical sibling. | |||||||||||
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; Flu, fludarabine; Cy, cyclophosphamide; TBI, total-body irradiation; Bu, busulfan; Mel, melphalan; E, extensive. | |||||||||||
| Khouri22 | 47 | Flu Cy Rituxan | 53 (33–68) | 45/2 | 100 | 60 | 83 | 85 | 15 | 4 | 60 |
| Rezvani18 | 62* | (Flu)TBI | 54 (33–66) | 30/32 | 67 | 36 | – | – | 42 | 20 | – |
| Rezvani18 | 26*8 | (Flu)TBI | 54(33–66) | 26/0 | 67 | 36 | 54 | 67 | 23 | 14 | 47 E |
| Rodriguez24 | 16 | Flu Mel | 51 (20–67) | NS | NS | 20 | 40 | 53 | – | 19 | 76 |
| 50 E | |||||||||||
| Hari28 | 88 | Various | 51 (21–70) | 88/0 | 69 | 35 | 55 | 62 | 28 | 17 | 62 |
| Vigouroux27 | 73 | Various | 51 (33–66) | 63/10 | 74 | 37 | 52 | 55 | 15 | 40 | 43 |
| 20 E | |||||||||||
| Kusumi26 | 45 | Various | 45( 32–66) | – | 67 | 24 | – | 75 | – | 20 | – |
| Robinson25 | 52 | Various | 46 (27–65) | 91%/9% | 85 | – | 54 | 65 | 31 | 21 | – |
| Armand23 | 13 | Flu Bu | 51 (34–64) | 6/7 | 82 | 26 | 59 | 81 | 0 | 41 | 61 |
| Author, ref . | N . | Conditioning . | Age, y (range) . | HLA SIB/0ther donor . | Chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| *Among 62 patients were several with transformed or aggressive follicular. | |||||||||||
| **Outcome in subset of patients with indolent (mostly follicular) and HLA identical sibling. | |||||||||||
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; Flu, fludarabine; Cy, cyclophosphamide; TBI, total-body irradiation; Bu, busulfan; Mel, melphalan; E, extensive. | |||||||||||
| Khouri22 | 47 | Flu Cy Rituxan | 53 (33–68) | 45/2 | 100 | 60 | 83 | 85 | 15 | 4 | 60 |
| Rezvani18 | 62* | (Flu)TBI | 54 (33–66) | 30/32 | 67 | 36 | – | – | 42 | 20 | – |
| Rezvani18 | 26*8 | (Flu)TBI | 54(33–66) | 26/0 | 67 | 36 | 54 | 67 | 23 | 14 | 47 E |
| Rodriguez24 | 16 | Flu Mel | 51 (20–67) | NS | NS | 20 | 40 | 53 | – | 19 | 76 |
| 50 E | |||||||||||
| Hari28 | 88 | Various | 51 (21–70) | 88/0 | 69 | 35 | 55 | 62 | 28 | 17 | 62 |
| Vigouroux27 | 73 | Various | 51 (33–66) | 63/10 | 74 | 37 | 52 | 55 | 15 | 40 | 43 |
| 20 E | |||||||||||
| Kusumi26 | 45 | Various | 45( 32–66) | – | 67 | 24 | – | 75 | – | 20 | – |
| Robinson25 | 52 | Various | 46 (27–65) | 91%/9% | 85 | – | 54 | 65 | 31 | 21 | – |
| Armand23 | 13 | Flu Bu | 51 (34–64) | 6/7 | 82 | 26 | 59 | 81 | 0 | 41 | 61 |
T cell–depleted conditioning (in vitro or in vivo).
| Author, ref . | N . | Conditioning . | T-depletion method . | Age, y (range) . | HLA SIB/other donor . | chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; Flu, fludarabine; Cy, cyclophosphamide; TBI, total-body irradiation; Mel, melphalan; BEAM, carmustine, etoposide, cytarabine, melphalan; E, extensive. | ||||||||||||
| Mandigers32 | 15 | Cy/TBI | – | 47 (30–57) | 15/0 | 67 | 36mo | 67 | – | 33 | 13 | 86 |
| 43 E | ||||||||||||
| Verdonck33 | 10 | Cy/TBI | Counter flow elutriation | 43 (31–55) | 10/0 | 30 | 25 mo | 70 | 70 | 27 | 0 | 35 |
| Juckett31 | 16 | CyTBI | T10B9 in vitro | 43 (27–62) | 65% | 31 | 64 mo | 62 | 62 | 25 | 12 | 35 |
| Morris36 | 41 | FluMel | Alemtuzumab in vivo | 49 (18–73) | 64% | 98 | 16 | 49 | 74 | 8 | 44 | 0 |
| Ingram35 | 44 | BEAM | Alemtuzumab in vivo | 48 (31–59) | 28/16 | 90 | 32 | 58 | 69 | 20 | 20 | 20 |
| Novitzky34 | 12 | Myeloablative | Alemtuzumab in vitro | 47 (21–57) | 12/0 | NS | 26 | – | 75 | 16 | 8 | 18 |
| Author, ref . | N . | Conditioning . | T-depletion method . | Age, y (range) . | HLA SIB/other donor . | chemo-sensitive, % . | Median follow-up, mo . | PFS, % . | OS, % . | TRM, % . | Relapse, % . | cGVHD, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS indicates progression-free survival; OS, overall survival; TRM, treatment-related mortality; cGVHD, chronic graft-versus-host disease; Flu, fludarabine; Cy, cyclophosphamide; TBI, total-body irradiation; Mel, melphalan; BEAM, carmustine, etoposide, cytarabine, melphalan; E, extensive. | ||||||||||||
| Mandigers32 | 15 | Cy/TBI | – | 47 (30–57) | 15/0 | 67 | 36mo | 67 | – | 33 | 13 | 86 |
| 43 E | ||||||||||||
| Verdonck33 | 10 | Cy/TBI | Counter flow elutriation | 43 (31–55) | 10/0 | 30 | 25 mo | 70 | 70 | 27 | 0 | 35 |
| Juckett31 | 16 | CyTBI | T10B9 in vitro | 43 (27–62) | 65% | 31 | 64 mo | 62 | 62 | 25 | 12 | 35 |
| Morris36 | 41 | FluMel | Alemtuzumab in vivo | 49 (18–73) | 64% | 98 | 16 | 49 | 74 | 8 | 44 | 0 |
| Ingram35 | 44 | BEAM | Alemtuzumab in vivo | 48 (31–59) | 28/16 | 90 | 32 | 58 | 69 | 20 | 20 | 20 |
| Novitzky34 | 12 | Myeloablative | Alemtuzumab in vitro | 47 (21–57) | 12/0 | NS | 26 | – | 75 | 16 | 8 | 18 |
Probabilities of (A) overall survival and (B) progression-free survival after HLA-identical sibling transplant and myeloablative conditioning in the 1990s. Data updated until November 2008 (data courtesy of CIBMTR).
Probabilities of (A) overall survival and (B) progression-free survival after HLA-identical sibling transplant and myeloablative conditioning in the 1990s. Data updated until November 2008 (data courtesy of CIBMTR).
(A) Overall and (B) progression-free survival stratified by disease transformation. (C) Relapse rate stratified by disease transformation. (D) Overall and progression-free survival in patients with indolent disease and related grafts (n = 26). Seattle non-Myeloablative Conditioning. Reprinted with permission from Rezvani et al.18
(A) Overall and (B) progression-free survival stratified by disease transformation. (C) Relapse rate stratified by disease transformation. (D) Overall and progression-free survival in patients with indolent disease and related grafts (n = 26). Seattle non-Myeloablative Conditioning. Reprinted with permission from Rezvani et al.18
Progression-free and overall survival (solid line) after reduced-intensity conditioning for patients with chemosensitive follicular lymphoma at MD Anderson Cancer Center. Reprinted with permission from Khouri et al.22
Progression-free and overall survival (solid line) after reduced-intensity conditioning for patients with chemosensitive follicular lymphoma at MD Anderson Cancer Center. Reprinted with permission from Khouri et al.22
Adjusted probability of progression-free survival after allogeneic transplants for follicular lymphoma by conditioning regimen: conventional myeloablative versus reduced-intensity (RIC). Reprinted with permission from Hari et al.28
Adjusted probability of progression-free survival after allogeneic transplants for follicular lymphoma by conditioning regimen: conventional myeloablative versus reduced-intensity (RIC). Reprinted with permission from Hari et al.28
Disclosures Conflict-of-interest disclosure: The author declares no competing financial intererests. Off-label drug use: None disclosed.
References
Author notes
Section of Hematology/Oncology, School of Medicine, University of Chicago, Chicago, IL