Abstract
Treatment of myeloma relapse needs to be individualized to reflect the effectiveness and toxicities of prior therapies, with consideration given to pragmatic issues such as the tempo of relapse, age of the patient, access to drugs and patient preference. In general, combination therapies have been associated with higher response rates and improved progression-free survival and may be preferable when a rapid response is required. Nevertheless, in a slower-tempo relapse it is unclear at this juncture whether sequencing of drugs or multi-agent combinations offer superior overall survival results. Fortunately, active novel agents that offer further possibilities for some myeloma patients have become available in clinical trials. In this review we will describe the various classes of novel drugs being tested and the pros and cons of preclinical testing, and will particularly focus on two agents with single-agent activity in myeloma: carfilzomib, a proteasome inhibitor, and pomalidomide, a member of the immunomodulatory class of drugs.
The availability of the proteasome inhibitor bortezomib and the immunomodulators (IMiDs) thalidomide and lenalidomide has dramatically improved survival for patients with myeloma.1,2 Nevertheless, single-agent activity for each of these drugs is only 25% to 50%,3–5 and the cellular mechanisms that exist in only a subset of patients with myeloma that confer responsiveness is far from clear. Furthermore, the majority of patients (even those who respond) develop resistance over time by mechanisms that also remain obscure. Consequently, despite extended relapse-free intervals the majority of patients with myeloma continue to suffer recurrent disease relapse and increasing degrees of drug resistance. Compounding these inherent weaknesses of efficacy, side effects also dominate in some patients, limiting the therapeutic window available. Thus, finding and developing newer agents with novel mechanisms of action or improved second generation agents with greater efficacy or less toxicity against known targets remains a pressing issue in seeking operational cure for this disease. This review highlights recent reports of agents directed against known targets with evidence of activity in clinical trials, covers agents directed against novel targets that are being explored in late stage clinical testing, and discusses the use of current preclinical models and their value.
Multi-agent Combinations Versus Therapy Rationing and Sequencing
At the time of relapse the question of multi-agent drug combinations versus therapy sequencing is most pressing in choosing a therapeutic strategy. Indeed, the relative value of rationing drugs versus using all active drugs in combination is for now an unanswerable question. As guidance however, single agent activity of all known active agents in myeloma is limited and, for the most part, combination therapies appear to confer higher response rates and longer survival than single-agent drugs (eg, bortezomib/doxil versus bortezomib6; lenalidomide and dexamethasone versus dexamethasone7; melphalan, prednisone and bortezomib versus melphalan and prednisone8; melphalan, prednisone and thalidomide versus melphalan and prednisone9). It is also true that 3- or even 4-drug combinations further increase reported response rates when contrasted with historic controls, with up to 80% to 90% response rates, at least in smaller phase II trials,10–12 but no randomized trial data yet provide guidance for the role of most these combinations in survival at relapse. Until such data are available, a personalized approach to patient care is most appropriate. Certainly the tempo of relapse and patient-specific criteria such as age may direct therapeutic choice, with slow biochemical relapse or older patients being more suitable for a trial of 1 or 2 drugs in combination, with the more aggressive 3- or 4-drug combination therapies being reserved for younger patients with more rapid relapse and/or higher-risk disease13 with greater risk of myeloma-induced end-organ damage.
Although combination therapies in relapse can be impressive in terms of early response, patients continue to relapse, and often quickly, when high genetic risk factors are present, thus highlighting the need to continue the pursuit of more and better agents.
Active Drugs Targeting Known Pathways
Second- and third-generation IMiDs, proteasome inhibitors and alkylating agents have recently reported early phase I and II results and look encouraging in terms of both activity and toxicity profile.
Proteasome Inhibitors
Bortezomib is the first proteasome inhibitor to be approved for treatment of myeloma and mantle cell lymphoma. Although one of the most active agents available for the treatment of myeloma only 40% to 50% of patients respond to single-agent treatment at the time of relapse.14 One recent study of over 600 patients confirmed that even when dexamethasone was added following an inadequate response to single-agent bortezomib, only 54% of patients responded.15
The target of bortezomib is the proteasome. The proteasome is responsible for the degradation of ubiquinated peptides in the cell. This activity is conferred by six catalytic active sites. Three of these form the constitutive proteasome, which is broadly expressed. The remaining three form a relatively hematopoietic restricted immunoproteasome.16 Although recent evidence suggests that the immunoproteasome is a valid target,17 most current proteasome inhibitors are less specific and target both the constitutive and the immunoproteasome.
New proteasome inhibitors have entered or will likely enter the clinic soon. These include carfilzomib (PR-171),18 salinosporamide (NPI-0052)19 and CEP18770.20 Although the ultimate target of proteasome inhibition is shared, these drugs differ somewhat from each other in chemistry and proteasome specificity, which may prove to confer clinical differentiation. These agents can currently be classified in three structural groups: boronic acid containing (bortezomib and CEP18770), beta lactone based (salinosporamide), and ketoepoxide based (carfilzomib). Although not yet in the clinic some of these agents or closely related analogues can be delivered orally and such oral proteasome inhibitors are being readied for phase I testing.21 Little clinical information is currently available on NPI-0052 or CEP18770 but early clinical data concerning carfilzomib have been presented.
Carfilzomib
Carfilzomib is a proteasome inhibitor from a new chemical class called peptide ketoepoxides which require an N-terminal threonine to bind; thus, binding is restricted to the proteasome.18 This highly selective mechanism eliminates the potential for off-target activity with other cellular proteases.
Carfilzomib is an irreversible inhibitor, and the recovery of proteasome activity depends entirely on new proteasome synthesis.16 Normal cells recover proteasome function through the synthesis of new proteasomes, whereas susceptible tumors cells undergo apoptosis. The period of time required to induce apoptosis varies among tumor cell types, with the most sensitive cells being those from hematological tumors.
In phase I studies, two consecutive-day dosing schedules were compared in patients with hematological malignancies. Carfilzomib was administered both on a 2-week cycle, with 5 days of daily dosing (QD × 5) followed by a 9-day rest period and on a 4-week cycle, with consecutive day 1/day 2 dosing (QD × 2) weekly for 3 weeks followed by a 12-day rest period. In both trials, greater than 80% proteasome inhibition was achieved at doses that were well tolerated. Clinical responses were seen across a range of doses in both studies, indicating that carfilzomib has a wide therapeutic index. In the QD × 5 trial, responses (minor responses [MR] or better) were seen in 8 of 19 patients with multiple myeloma (MM) and 1 with Waldenström macroglobulinemia. The population of responding patients was heavily pretreated (range of 4 to 10 prior therapies), and responses occurred in patients who were refractory to bortezomib. Responses were durable, lasting over one year in some cases, and there was an apparent reduction in the incidence of the painful peripheral neuropathy that is a dose-limiting side effect seen with bortezomib treatment.
Two phase II clinical trials of carfilzomib have been reported.22,23 Patients on both studies were treated initially with 20 mg/m2 carfilzomib on the QD × 2 schedule.
In the first 46 patients treated at 20 mg/m2 carfilzomib on a trial for relapsed refractory patients, preliminary efficacy data showed a clinical benefit response of 26% (10 of 39), including 7 PRs and 3 MRs and the classical overall response rate (CR, VGPR, PR) was 18%. Additionally, 41% (16 of 39) of these highly refractory patients experienced stable disease (SD) with the majority a durable SD of longer than 4 months. The median duration of response for the MR + PR patients was 7.4 months, with 8 of 10 patients achieving a response during Cycle 1. Five of the responding patients had received and progressed on a bortezomib-containing regimen as their last treatment. All responses were durable even though these patients had failed an average five prior therapies, including prior bortezomib and an IMiD agent. The median TTP (n = 46) was 5.1 months. This TTP is similar to that observed with bortezomib in both the SUMMIT trial conducted in relapsed and refractory patients (median TTP = 7 months) and the APEX trial conducted in patients who had relapsed (median TTP = 6.2 months).
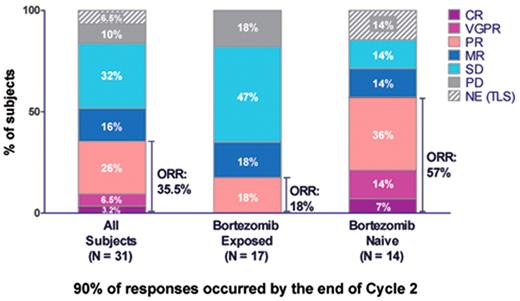
In a less heavily treated patient population who had relapsed from one to three prior therapies 31 patients have been analyzed to date (Figure 1 ); 14 were bortezomib naïve (45%), and 17 patients (55%) had received prior bortezomib treatment. In the bortezomib-naïve patients, the ORR was 57% (8/14), including 1 CR, 2 VGPRs, and 5 PRs. The median TTP in this population was 11.1 months. In patients who had received bortezomib prior to enrollment in this study, the ORR was 18% (3 of 17 patients; all PRs). Additionally, 3 patients (18%) achieved an MR and 8 patients (47%) had SD. Although the response rate was reduced relative to the response rate in bortezomib-naïve patients, the median TTP in the patients who were previously exposed to bortezomib was still 8.3 months. Seven patients (41%) were progression free when last reported and 6 patients had completed 12 cycles of carfilzomib, indicating that prior treatment with bortezomib does not preclude clinical benefit with carfilzomib treatment.
The primary Grade 3/4 hematological events in this population were anemia (37%) and thrombocytopenia (26%), and Grade 3/4 neutropenia was only 4.3%. The primary non-hematological adverse effects were fatigue and upper respiratory and gastrointestinal events. There was a low rate of treatment-emergent peripheral neuropathy (2.2% Gr 3) in a population in which 78% had Grade 1/2 neuropathy at baseline and 98% had a history of drug- or disease-related neuropathy. Some non-cumulative elevations in creatinine were seen especially with the first dose of drug. Two cases of what appeared to be tumor lysis were observed, but no further cases have been seen since prophylaxis was routinely introduced.
Immunomodulators
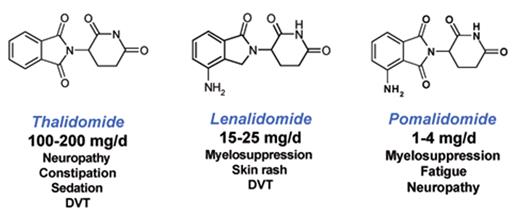
The third IMiD to enter the clinic was pomalidomide (CC4047).24,25 As diagrammed in Figure 2 , Pomalidomide is remarkably similar to thalidomide and lenalidomide.
Pomalidomide is a more potent IMiD, at least when measured in in vitro studies.26–28 IMiDs have many biochemical effects but the mechanism of action is for the most part still poorly understood. Postulated mechanisms of action include inhibition of wnt signaling, blocking signaling through nuclear factor-kappa B, pro-apoptotic properties via the caspase-8/death receptor pathway. anti-angiogenic effects, downregulation of tumor necrosis factor, augmentation of natural killer cell activity and stimulation of cytotoxic T-cells.26,27,29–34 Indeed data suggest pomalidomide is the most potent of the IMiDs.26,27,29
Phase I trials established pomalidomide as well tolerated in doses ranging from 1 to 5 mg/day.25 The first phase II trial of pomalidomide combined with low-dose dexamethasone (Pom/dex) in patients with relapsed or refractory MM has now been reported.35 Sixty patients were enrolled. Pomalidomide was given orally at a dose of 2 mg daily on days 1 through 28 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15, 22 of each cycle. Aspirin 325 mg orally daily was given as prophylaxis for thromboembolic disease. Responses were recorded using the criteria of the International Myeloma Working Group.
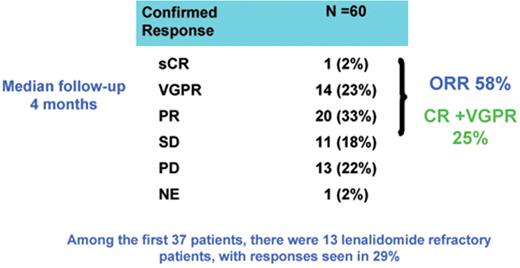
Thirty-eight patients achieved objective response (63%) including CR in 3 patients (5%), VGPR in 17 patients (28%), PR in 18 patients (30%) (Figure 3 ). The CR + VGPR rate was 33%. Responses were seen in 8 of 20 lenalidomide-refractory patients (40%), 6 of 16 thalidomide-refractory patients (37%) and 6 of 10 bortezomib refractory patients (60%). Responses were seen in 11 of 13 (85%) patients with high risk cytogenetic or molecular markers. Toxicity consisted primarily of myelosuppression. Grade 3 or 4 hematologic toxicity consisted of anemia in 3 patients (5%), thrombocytopenia in 2 patients (3%) and neutropenia in 21 (35%). One patient (1.6%) had a thromboembolic event. In conclusion the combination of pomalidomide and dexamethasone is active in the treatment of relapsed MM, demonstrating high response rates in patients refractory to other novel agents.
Alkylators
The alkylating agent bendamustine has structural similarities to both alkylating agents and purine analogs and is non-crossresistant with alkylating agents and other drugs in vitro.36 Superiority over chlorambucil in previously untreated patients with chronic lymphocytic leukemia (CLL) led to its recent approval for this disease in the United States. Bendamustine is also active in patients with MM.37,38 Pönisch et al38 randomly assigned 131 patients to bendamustine (150 mg/m2 days 1 and 2) or melphalan (15 mg/m2 day 1) every 4 weeks; both arms received prednisolone (60 mg/m2 daily on days 1 through 4). Cross-over was permitted within 3 months for progression. The ORR was 75% for the bendamustine-prednisolone arm and 70% for melphalan-prednisolone. However, bendamustine achieved CRs in 32% compared with 13% with melphalan (P = .007), with a shorter time to maximum response with bendamustine: 6.8 cycles for bendamustine-prednisolone, but 8.6 cycles with melphalan-prednisolone (P < .02). TTF was longer for bendamustine at 14 months compared with 10 months for melphalan (P < .02), with no difference in overall survival. The toxicities of the two arms were comparable. Despite this preliminary evidence of activity this study was underpowered, the CR rate to melphalan was higher than generally expected and, since dosing with intravenous melphalan is not standard, larger confirmatory studies would be required to demonstrate an advantage over melphalan in this setting. Further evaluation of bendamustine in MM appears warranted, and this agent is currently being tested in combination with other, active agents, such as bortezomib.
Novel Pathway Drugs and Early Clinical Results
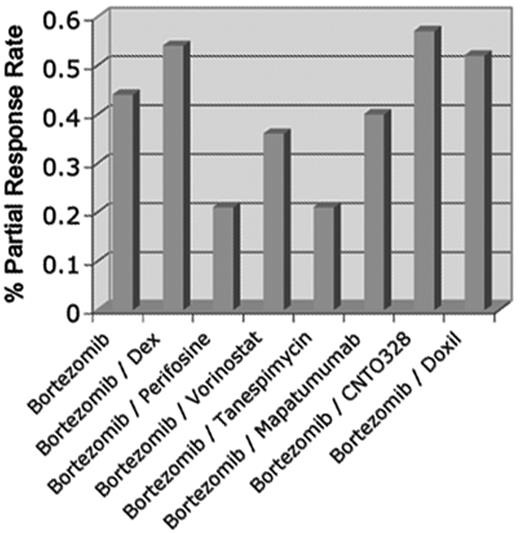
A review of the medical literature reveals over 180 different drugs in which preclinical studies have indicated a rationale to proceed to clinical testing in myeloma. Of these approximately 30 have entered trials. Clinical data on only a handful of these drugs are currently available. Unfortunately, apart from the proteasome inhibitors, alkylators and IMiDs described above, none of the trials of drugs targeting new pathways has yet reported significant single-agent activity. Some of these new agents have also been combined with bortezomib or lenalidomide in phase Ib/II testing, but a significant improvement in response rates when combined with active agents is difficult to discern (see below). One lesson from these results has been the great difficulty in cross-comparison of studies when different patient populations with variable numbers of prior therapies and degrees of “resistance” are studied at relapse (Figure 4 ). A very relevant point to bear in mind when attempting such an analysis is that current definitions of refractory disease allow for patients who may still be responsive to, as an example, bortezomib (with which a new drug is being combined) but have progressed within 60 days of discontinuation of bortezomib-therapy. Some of these patients will presumably still initially respond to a bortezomib containing cocktail; thus, defining the contribution of a new drug becomes vexing. Nevertheless, with these reservations in mind responses have indeed been seen when new drugs are combined in patients defined as “resistant” to bortezomib or lenalidomide alone, and this may then constitute evidence of the ability of the new drug to synergize or overcome resistance when used in combination. Furthermore, a high percentage of patients have obtained stabilization of disease when treated with new drugs; indeed, some of these drugs may have a more cytostatic than cytotoxic effect. Some examples of drugs targeting novel pathways which have been reported are discussed here.
AKT Inhibitors
Perifosine is an alkylphospholipid that modifies intracellular growth signal transduction pathways. Importantly, perifosine inhibits AKT activity.39 Since AKT plays a crucial role in MM cell survival, several studies have shown that inhibition of AKT activity by perifosine could represent a novel therapeutic strategy in MM.39 These data provided a rationale for a clinical trial with perifosine alone and in addition to dexamethasone showing an MR in 1 of 48 patients and stable disease in 22 patients taking perifosine as a single agent. In 31 patients treated with perifosine with added dexamethasone PR, MR and SD were achieved in 4, 8 and 15, respectively.40 In addition, perifosine with bortezomib, with or without dexamethasone, or perifosine with lenalidomide plus dexamethasone is currently under evaluation, with preliminary reported response rates of 33% and 66%, respectively.41,42
Heat-shock-protein (Hsp) Inhibitors
The first Hsp90 inhibitor to enter clinic was the geldanamycin (GM) derivative 17-allylamino-17-desmethoxy-geldanamycin (17-AAG).46 A cremophor-containing formulation of 17-AAG has been developed (tanespimycin, KOS-953). In a phase Ib dose-escalating trial that evaluated tanespimycin with bortezomib in patients with relapsed, refractory multiple myeloma in the bortezomib-naive group, the ORR (CR, PR, and MR) was 47% (9 out of 19 evaluable patients), including 2 CR, 1 near-CR, 2 PR, and 4 MR. In the bortezomib pre-treated group, the ORR was 47% (7 of 15 evaluable patients; 1 CR, 2 PR, and 4 MR). In the bortezomib-refractory group, the ORR was 17% (3 of 18 evaluable patients; 3 PR).47,48
Tanespimycin has entered late-stage clinical testing and other potentially more potent Hsp inhibitors such as AUY922 are now in phase I clinical testing.49
Histone Deacetylase Inhibitors
Histone acetylation modulates gene expression, cellular differentiation and survival; it is regulated by the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDAC inhibition induces differentiation and/or apoptosis in transformed cells, and it has been hypothesized that HDAC inhibition could function as a master switch that could simultaneously affect multiple pathways critical for survival of MM cells. Based on preclinical studies,50,51 a phase I dose escalation of oral vorinostat (200, 250 and 300 mg p.o. b.i.d. for 5 consecutive days followed by 2 days of rest) was administered in monthly cycles in patients with relapsed/refractory MM. In 7 patients with relapsed refractory MM, 2 MRs were observed and 2 patients had SD. In combination with bortezomib, two pilot studies have been done with vorinostat (16 and 17 patients in each), with 8 patients (50%) achieving PR or near-CR and 6 patients with disease stabilization in the first study and 4 with PR (23%), 2 with MR and 11 with SD in the second.52–55
A second HDAC inhibitor is panobinostat (LBH589). In a phase II trial the safety, tolerability, and ORR of 20 mg/day of oral panobinostat given on a Monday/Wednesday/Friday (MWF) dosing schedule were assessed.56 Thirty-eight patients were enrolled. The median number of prior therapies was 5 (2–12). The most common Grade 3/4 adverse effects were cytopenias, with neutropenia, thrombocytopenia, and anemia in 32%, 26%, and 16% of patients, respectively. Only 2 patients (5%) showed any response to single-agent drug at this dose (1 VGPR and 1 MR).
In a phase Ib combination study 14 patients have been reported in two cohorts: Cohort 1 (10 mg panobinostat + 1.0 mg/m2 bortezomib, n = 7) and Cohort 2 (20 mg panobinostat + 1.0 mg/m2 bortezomib, n = 7). Disease status included 9 of 14 patients refractory at entry, defined as progressing within 60 days of last therapy. Of the 14 patients, 1 immunofixation-negative CR, 1 VGPR, and 3 PR were seen, although the addition of dexamethasone was required for 3 (1 CR and 2 PR) of these 5 patients due to poor response to cycle one.57
Large phase III trials are now testing some of the agents that target novel pathways in myeloma, such as vorinostat, tanespimycin, and perifosine, as described above. Many other drugs have been studied in phase I and phase II testing but to date no major therapeutic advance has crystallized.
Advances in Preclinical Testing and Understanding Mechanism of Action
Overall the poor positive predictive value of preclinical testing exemplified by the data above suggest a need for enhanced preclinical models or more aggressive mechanisms by which to quickly screen and discard inactive agents in early-phase clinical testing. In the case of the negative predictive value of preclinical testing it is noteworthy that thalidomide (as an example) is singularly inactive in preclinical testing and would likely never have been tested in patients had preclinical studies been the sole guides for the decision to proceed to clinical testing.
Clearly then, while clinical advances continue to be made, progress has been mostly serendipitous: the activity of proteasome inhibitors was discovered in a broad phase I trial for hematologic malignancy and thalidomide activity was discovered when it was used off label for its putative anti-angiogenic effects. Thus a refinement of preclinical testing seems in order and overdue. Going forward, new animal models and use of pharmacogenomic strategies may offer some advance in selecting therapies for clinical testing in a more rational fashion.
As one example of a novel preclinical animal model Chesi et al58,59 have successfully generated the first immunocompetent transgenic mouse model of MM, Vk*MYC, in which sporadic MYC activation in the germinal center is AID dependent and mediated by the B-cell somatic hypermutation process, such that only post-switch, somatically mutated B cells can overexpress MYC. All of Vk*MYC mice develop monoclonal PC expansion by 50 weeks of age, which is manifested by high levels of serum IgGs and major M-spikes by SPEP. The PCs in Vk*MYC mice are fully differentiated, somatically mutated, have a very low proliferation index and are found exclusively in the bone marrow. Associated with a long lifespan (medium survival = 661 d), Vk*MYC mice develop anemia and diffuse bone disease (osteoporosis with low bone mineral density), with sporadic occurrence of lytic bone lesions and hind limb paralysis. The phenotypic fidelity of the Vk*MYC mice demonstrates a therapeutic similarity with the human condition (at least in the small number of drugs studied to date), as Vk*MYC mice show response to known clinically active anti-myeloma drugs (bortezomib, carfilzomib, melphalan, dexamethasone) but not to drugs that have shown no clinical activity as single agents (vincristine, hydroxyurea, fludarabine). Furthermore, acquired bortezomib resistance could efficiently be induced in Vk*MYC mice after only four cycles of suboptimal treatment, and studies are in progress to identify genetic events associated with bortezomib resistance. Genomic data suggest that the genomic complexity in this inbred strain of transgenic mice is significantly less then in patients with MM, simplifying the search for critical loci involved in MM tumorigenesis and drug resistance.
Summary
Treatment for myeloma relapse needs to be individualized to reflect prior drug exposures, prior drug toxicities and responsiveness, geography, age, tempo of relapse and genetic risk. In general, multiple drug combinations appear to be associated with higher overall and complete response rates and would generally be favored where rapid disease control is required. Nevertheless, it is unclear at this time whether sequencing drugs or using multiple-agent combinations is superior in terms of survival. New drugs are showing promise in clinical trials, specifically carfilzomib, pomalidomide and bendamustine, while a number of other drugs targeting novel molecular mechanisms are in late-stage clinical testing. Newer animal models are now available that may allow the refinement of preclinical testing, thus making the bench to bedside process more efficient and predictive of clinical success. Numerous other investigational agents are entering early-phase clinical testing, and it seems probable that the therapeutic arsenal for myeloma will continue to expand and patient outcomes continue to improve.
Single-agent activity of carfilzomib in patients who have received one to three prior therapies. Overall response rates are shown along with responses in bortezomib-exposed and bortezomib-naïve patients.
Single-agent activity of carfilzomib in patients who have received one to three prior therapies. Overall response rates are shown along with responses in bortezomib-exposed and bortezomib-naïve patients.
Thalidomide, lenalidomide and pomalidomide share similar chemical structures yet have different clinical profiles.
Thalidomide, lenalidomide and pomalidomide share similar chemical structures yet have different clinical profiles.
Activity of pomalidomide with low-dose dexamethasone in patients who have received one to three prior therapies. sCR indicates stringent complete remission; PR, partial response; VGPR, very good PR; SD, stable disease; PD, progressive disease; NE, not evaluable.
Activity of pomalidomide with low-dose dexamethasone in patients who have received one to three prior therapies. sCR indicates stringent complete remission; PR, partial response; VGPR, very good PR; SD, stable disease; PD, progressive disease; NE, not evaluable.
Representative examples of response rates for bortezomib and bortezomib combination trials with novel agents in relapsed or relapsed and refractory myeloma highlight the difficulty in comparing across trials with varying eligibility but also point out that few combinations with novel agents appear to conclusively show major benefit when response rate alone is used as the major criterion.
Representative examples of response rates for bortezomib and bortezomib combination trials with novel agents in relapsed or relapsed and refractory myeloma highlight the difficulty in comparing across trials with varying eligibility but also point out that few combinations with novel agents appear to conclusively show major benefit when response rate alone is used as the major criterion.
Disclosures Conflict-of-interest disclosure: The author is a consultant for Takeda-Millennium, Celgene, Novartis, and Amgen; receives research funding from Takeda-Millenium; and receives honoraria from Genzyme, Celgene, Millennium, and Proteolix. Off-label drug use: None disclosed.
References
Author notes
Hematology and Oncology, Mayo Clinic Arizona, Scottsdale, AZ