Abstract
Although classical Hodgkin lymphoma (HL) is considered one of the most curable human cancers, the treatment of patients with relapsed and refractory disease, especially those who relapse after autologous stem cell transplantation, remains challenging. Furthermore, because the median age of the patients is in the mid-30s, the impact of early mortality on the number of years lost from productive life is remarkable. Patients with HL whose disease relapses after stem cell transplantation are rarely cured with current treatment modalities. New drugs and novel treatment strategies that are based on our understanding of the disease biology and signaling pathways are needed to improve treatment outcome for these patients. This review will focus on emerging new treatment modalities that are currently under investigation for patients with relapsed classical HL.
Hodgkin lymphoma (HL) is a rare human cancer with an estimated 8220 new cases in the United States in 2008.1 In spite of its high cure rate, patients who are not cured with front-line or second-line therapy, including stem cell transplantation, have an estimated median survival of less than 3 years.2 As the median age of this patient population is the mid-30s, the impact of early mortality on the number of years lost from productive life is remarkable. Because of the relatively rare incidence and high cure rate, the development of new drugs for the treatment of relapsed HL has been very challenging. In fact, no new drugs have been approved for HL by the US Food and Drug Administration (FDA) in more than 30 years. Thus, drug development in this area will address a significant unmet medical need.3
With recent advances in our understanding of HL pathology, biology, and immunology, several therapeutic targets have been identified and are currently under preclinical and clinical investigation.3,4 For example, the malignant Hodgkin and Reed-Sternberg (HRS) cells of HL express several receptors that belong to the tumor necrosis factor (TNF) receptor family, including pro-survival receptors (CD30, CD40, TACI, BCMA, and RANK) and pro-death receptors (Fas, and TRAIL death receptors 1 and 2).5,6 The pro-survival receptors share similar biological functions, including induction of cytokine and chemokine secretion, and activation of shared signaling pathways such as NF-κB, ERK/MAPK, and PI3-kinase/Akt/mTOR.7,8 These receptors are currently being explored for novel treatment strategies by using a variety of naked and conjugated monoclonal antibodies (Figure 1 ). Furthermore, signaling pathways triggered by these receptors and other intracellular proteins can now be therapeutically inhibited by a variety of small molecules. This review will focus on promising new drugs that are currently in clinical trials for the treatment of patients with relapsed classical HL.
Targeting Surface Receptors and Antigens
CD30
The dense expression of CD30 by HRS cells coupled with its highly restricted expression makes it an obvious target for therapeutic monoclonal antibody.9,10 In addition to its transmembrane form, CD30 is also shed in a soluble form that can be detected in sera of patients with anaplastic large cell lymphoma (ALCL) and HL.11 –13 Thus, unlike CD20, which is rarely shed in the serum, the presence of soluble CD30 may attenuate the therapeutic efficacy of anti-CD30 antibodies.
Results from two clinical studies using first-generation naked anti-CD30 monoclonal antibodies in patients with relapsed HL have been disappointing, perhaps reflecting their poor antigen binding and/or effector cell activation properties (Table 1 ).14,15 It is also possible that the activity of these antibodies was neutralized in vivo by circulating soluble CD30. Several strategies are currently being developed to improve the efficacy of therapeutic anti-CD30 antibodies. For example, to bypass the neutralizing effect of soluble CD30, it is now possible to engineer an anti-CD30 antibody that can selectively bind to the transmembrane form.16 However, this approach has not been clinically tested. A different approach would be to improve antigen binding and Fcγ receptor IIIA affinity and specificity of the antibody. To do so, the chimeric anti-CD30 antibody cAC10 was humanized by optimizing human string content to generate the novel Xmab2513 antibody, resulting in a significant improvement in binding to CD30 antigen and to the FcγRIIIA receptor on effector cells.17,18 In vitro experiments demonstrated that XmAb2513 had more potent antiproliferative activity compared with cAC10 and 5F11 antibodies. Based on this promising data, a phase I study of Xmab2513 is currently enrolling patients in the United States, and encouraging preliminary results were recently reported.19 To date, 17 patients received escalating doses ranging between 0.3 mg/kg and 12 mg/kg, given every 2 weeks, and the maximum tolerated dose (MTD) has not yet been reached. One patient treated at the 9 mg/kg dose level achieved a partial remission, suggesting that this novel antibody may indeed be more effective than the first-generation anti-CD30 antibodies.
In an alternate strategy, the anti-CD30 antibody cAC10 was conjugated to a synthetic anti-microtubule agent, monomethyl auristatin E (MMAE), resulting in a novel immunotoxin conjugate called SGN-35.20 Using this approach, the antibody is used as a delivery system and therefore it can demonstrate potent therapeutic efficacy independent of ADCC. In vitro experiments using a variety of HL and ALCL cell lines demonstrated that SGN-35 had potent and selective in vitro activity, with IC50 ranging from 4 to 35 ng/ mL for CD30+ cell lines, compared with IC50 greater than 1000 ng/mL for the CD30− cells. SGN-35 was recently evaluated in two phase I clinical trials in patients with relapsed HL and ALCL. In the first study, SGN-35 was administered by intravenous infusions every 3 weeks.21 A total of 45 patients were treated with escalating doses ranging between 0.1 and 3.6 mg/kg. Forty-two had relapsed HL, of whom 76% were previously treated with high-dose therapy and autologous stem cell transplant (ASCT). The treatment was reasonably well tolerated, although dose-limiting toxicities (neutropenia and hyperglycemia) were observed at doses higher than 1.8 mg/kg. Seventeen (37%) patients achieved partial or complete remissions, and 88% of the patients had documented tumor reductions.21 A second phase I study evaluated the safety and efficacy of the SGN-35 given on a weekly schedule.22 Preliminary results demonstrated that doses up to 1.2 mg/kg can be safely administered. Of 17 evaluable patients, 7 achieved CRs and 1 achieved a PR, for an overall response rate of 47%. Based on this encouraging clinical activity and favorable safety profile, SGN-35 is currently being evaluated in two phase-II pivotal trials seeking FDA approval in patients with relapsed HL and ALCL.
CD40
CD40 receptor is more widely expressed compared with CD30 and CD20. It is physiologically expressed on hematopoietic (B cells, monocytes, dendritic cells), and non-hematopoietic (epithelial, and endothelial) cells.9,10 CD40 is also expressed on malignant cells originating from CD40-expressing benign cells, including B-cell lymphoma, HRS cells of HL, and breast carcinoma.9,26,27 Activation of CD40 receptor in HRS cells induces NF-κB, cytokine and chemokine secretion, and upregulated survival proteins. Although HRS cells do not express CD40L (CD154), they receive CD40L signals from the surrounding reactive cells, including B cells, T cells, and eosinophils.28,29 Thus, therapeutic targeting of CD40 is more complex than targeting CD30 or CD20. To date, two anti-CD40 antibodies (SGN-40 and HCD122) are being evaluated in patients with CD40-expressing lymphoid malignancies, but only the HCD122 study is enrolling patients with HL.30 –34 While the single-agent activity of anti-CD40 antibody therapy is currently unknown, it is likely that this antibody will have a better therapeutic value when given with other drugs. In fact, several in vitro studies demonstrated potential synergy between anti-CD40 blocking antibodies and a variety of chemotherapy drugs.
TRAIL (Apo2L) and Its Receptors
Tumor necrosis factor apoptosis-inducing ligand (TRAIL) is a death protein that is primarily expressed by activated T cells and NK cells.10 TRAIL has four exclusive receptors: TRAIL-R1 (DR4), TRAIL-R2 (DR5, KILLER, TRICK2), TRAIL-R3 (DcR1, TRID, LIT), and TRAIL-R4 (DcR2 (TRAIL-R4, TRUNDD).35 TRAIL-R1 and TRAIL-R2 are death receptors that primarily recruit the death domain-containing adaptor protein FADD (Fas-associated death domain), which recruits the apical caspases 8 and 10 to initiate apoptosis. TRAIL-R3 and TRAIL-R4 are decoy receptors.36 TRAIL has a wide range of activity against primary and cultured tumor cells.37 HL cell lines express TRAIL receptors R1, R2, and R4, but not R3.8 Both APO2L/ TRAIL protein and agonistic anti–TRAIL-R1 and –TRAIL-R2 antibodies have been shown to induce cell death in HL cell lines.8,38 Results from a phase II study using an agonistic anti–TRAIL-R1 monoclonal antibody in patients with relapsed non-Hodgkin lymphoma (NHL) demonstrated excellent safety profile and a promising clinical activity, especially in patients with follicular lymphoma.39 Although there is currently no single-agent activity for TRAIL or agonistic antibodies to TRAIL-R1/R2 in patients with HL, an ongoing phase I clinical trial combining the agonistic anti–TRAIL-R2 (AMG655) with vorinostat or bortezomib is currently enrolling patients with both HL and NHL. Histone deacetylase (HDAC) inhibitors and proteasome inhibitors have been shown to potentiate death-receptor mediated apoptosis by modulating the expression of TRAIL-R1 and R2, cFLIP, Bcl-2 family, and NF-κB.
Interleukin (IL)-13 and IL-13 Receptors
The rationale for targeting the IL-13/IL-13R pathway is based on data demonstrating that cultured and primary HRS cells express both IL-13 and IL-13Rα-1, and neutralizing antibody to IL-13 inhibited the growth of HL cell lines in vitro.40,41 IL-13 and IL-13Rα-1 are found to be expressed in more than 70% of classical HL lymph nodes, and approximately 10% of newly diagnosed patients with HL and 16% of patients with relapsed HL have detectable levels of IL-13 in their sera.42 With this background, a phase I clinical trial using a fully human anti-IL-13 monoclonal antibody (TNX-650) has recently completed enrollment in patients with relapsed HL, and the results should become available in the near future.
CD80 (B7.1)
CD80 is a membrane-bound costimulatory molecule that is involved in regulating T-cell activation.43,44 In healthy individuals, CD80 is transiently expressed by activated B cells, dendritic cells, and T cells.45 CD80 is constitutively expressed by follicular B-cell lymphoma and HRS cells of HL, making it a potential therapeutic target.46,–50 Preclinical studies demonstrated that the anti-CD80 antibody galiximab, a primatized, anti-CD80 (IgG1) monoclonal antibody with human constant region and primate (cynomologus macaque) variable region, can inhibit lymphoma cell proliferation by antibody-dependent cell-mediated cytotoxicity (ADCC). Galiximab was recently evaluated in patients with relapsed B-cell follicular lymphoma and showed modest, but promising clinical activity.51 Galiximab is currently being evaluated in a phase II clinical trial in patients with relapsed HL.
Targeting Intracellular Survival Pathways with Small Molecules
HRS cells aberrantly express a variety of pro-survival proteins, such as NF-κB, Jak/STATs, Akt/mTOR, Notch-1, and ERK, that can be targeted by small molecules.7,8,52 These proteins can be targeted with selective small molecule inhibitors (Jak2, mTOR, and Bcl2 family inhibitors), or by broad inhibitors that modulate several unrelated molecules HDAC, proteasome, and heat shock protein [HSP]-90 inhibitors) (Figure 1 ).
Histone Deacetylases (HDAC) Inhibitors
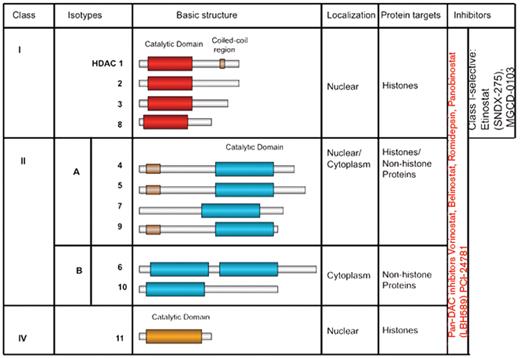
It is well established that post-transcriptional histone modification plays an important role in regulating gene transcription and is mediated by a several of enzymes, including histone acetyltransferases (HATs) and histone deacetylation (HDACs).53 These enzymes mediate acetylation and deacetylation of specific lysine amino acid residues on histone and non-histone proteins, including transcription factors (p53, STAT3, MYC, GATA-1, GATA-2, E2F, NF-κB, nuclear receptors, HIF-1α, and TEL), α-tubulin, and HSP90.53,54 The balance between HATs and HDACs is critical for regulating the expression and the functional status of a variety of proteins that are involved in cell proliferation, survival, angiogenesis, and immunity.55,–57 To date, 18 HDACs have been identified in humans, and are grouped in two major categories: zinc-dependent HDACs and NAD-dependent HDACs.58,59 Furthermore, HDACs are classified into four major classes: Class I (HDAC 1, 2, 3, 8, and 11); Class II (HDAC 4, 5, 6, 7, 9, and 10); Class III (SIRT 1–7), and Class IV (HDAC 11) (Figure 2 ). Class III is NAD-dependent, whereas classes I, II, and IV are zinc dependent.
At the present time, several clinical grade pharmacologic inhibitors of the zinc-dependent HDACs are available for clinical trials, but only one inhibitor (vorinostat, SAHA) has been approved by the FDA. Vorinostat and panobinostat (LBH589) inhibit HDAC classes I and II (pan-HDAC inhibitors). MGCD0103 and etinostat (SNDX-275, formerly MS-275) preferentially inhibit class I HDACs (isotype selective HDAC inhibitors).
Although HRS cells are of B-cell origin, they infrequently express B-cell antigens.60 This loss of B-cell phenotype has been reported to be epigenetically regulated and may be therapeutically reversible.61,62 Several HDAC inhibitors have antiproliferative activity in HL-derived cell lines in vitro. In a recent study, vorinostat was shown to induce cell cycle arrest and apoptosis in HL cell lines and to synergize with chemotherapy.63 Furthermore, vorinostat inhibited STAT6 phosphorylation and transcription in HL cell lines, an effect that was associated with a decrease in the expression and secretion of Th2-type cytokines and chemokines, including thymus and activation-regulated chemokine (TARC/CCL17) and IL-5, and an increase in Th1-type cytokines/chemokines, including a profound increase in IP-10 levels.63 Finally, both HDAC inhibitors, alone or in combination with hypomethylating agents, have been shown to induce cancer testis antigen (CTA) expression, including MAGE, SSX, and NY-ESO-1 family members in a variety of tumors, and therefore may induce favorable anti-tumor immune response in vivo.64
Several HDAC inhibitors are currently being evaluated for the treatment of relapsed HL (Table 1 ). MGCD-0103 is a novel oral nonhydroxymate benzamide-based HDAC inhibitor that selectively inhibits HDAC 1 and 2 (and to a lesser extent, 3 and 11) isoforms.65 Its IC50 for inhibiting recombinant HDAC1 activity is 0.082 mM compared with > 30 mM for HDAC6.66,67 The safety and efficacy of MGCD0103 given orally 3 times per week (85 mg to 110 mg starting doses) was recently evaluated in a phase II study in patients with relapsed and refractory HL. Patients were allowed to receive therapy for 1 year in the absence of disease progression or prohibitive toxicity. Of the 20 patients who were treated with 110-mg dose level, 7 (35%) patients achieved partial or complete remissions. However, this dose level was poorly tolerated resulting in dose interruptions and reductions, and discontinuation of therapy after a median of 4.5 months. Subsequently, the study was revised to allow a lower starting dose of 85 mg at the same schedule. Three of the 10 (30%) patients enrolled on the reduced dose achieved partial remissions. Furthermore, grade 3 and 4 toxicity (mainly fatigue, with no significant hematologic toxicity) was reduced to 20%. Overall, 80% of the 30 evaluable patients had some decrease in their tumor measurements. Although none of the patients developed significant EKG abnormalities, two patients developed significant pericardial effusions requiring discontinuation of therapy. Collectively, this data indicate that class I HDAC inhibitors have a potential therapeutic value in patients with HL.
Two pan-HDAC inhibitors were recently evaluated in patients with relapsed HL. In the first study, panobinostat was evaluated in a phase I trial in patients with hematologic malignancies that also included patients with relapsed HL.69 Five of 13 (38%) patients achieved partial remissions. The most common side effects were fatigue, thrombocytopenia, nausea, and diarrhea. Based on this promising clinical activity, a large international phase II study of panobinostat in relapsed HL is now enrolling patients to confirm these results.70 In a second study, the Southwest Oncology Group (SWOG) conducted a phase II trial of vorinostat in patients with relapsed HL.71 Twenty-five patients were treated with 200 mg vorinostat given orally twice per day for 14 days every 21-day cycle. Unlike MGCD0103 and panobinostat, vorinostat produced modest clinical activity, as only 1 patient (4%) achieved a partial remission.
Collectively, both class I and pan-HDAC inhibitors demonstrated meaningful clinical activity in heavily pre-treated patients with relapsed classical HL. Future studies should examine the pattern of HDAC enzyme expression in HL to determine whether a certain pattern of expression may predict response to therapy. Furthermore, because HDAC inhibitors can modulate a variety of survival proteins, combination regimens with HDAC inhibitors should be investigated.
PI3K/Akt/mTOR
The phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway (Figure 3 ) is one of the most aberrantly activated survival pathway in cancer, making it an important target for drug development.72,73 This pathway is negatively regulated by the tumor suppressor protein PTEN. Unlike most cancers, in which PI3K activation is frequently associated with PTEN deletion or mutation, other mechanisms have been reported to activate this pathway in HL, including activation of CD30, CD40, and RANK receptors, mutations in the p85a subunit of the PI3K, and inactivation of PTEN function by phosphorylation.74,–79 In vitro experiments demonstrated that inhibition of PI3K, Akt, or mTOR by various small molecules can induce cell cycle arrest, autophagy, and apoptosis in HRS-derived cell lines in vitro.8081,82 In addition to a direct antitumor effect, mTOR inhibitors may induce clinical responses by enhancing the immune response and inhibiting angiogenesis.83,84
The therapeutic value of inhibiting the PI3K/Akt/mTOR axis has been recently studies using the oral mTOR inhibitor everolimus (Figure 3 ).85 Fifteen evaluable patients with relapsed HL were treated with daily doses of 10 mg everolimus, of whom 7 (47%) patients achieved partial responses (Table 1 ). Grade 3 adverse events included thrombocytopenia and anemia. If confirmed in a larger number of patients, everolimus may become one of the most active agents in relapsed HL. Because HRS cells frequently demonstrate aberrant and simultaneous activation of several survival pathways, including NF-κB, ERK, PI3K/Akt (Figure 3 ), rationally designed combination strategies will be required to improve the response rate and to prolong the response duration of mTOR inhibitors. In vitro experiments suggested that mTOR inhibitors may synergize with chemotherapy, PI3K inhibitors, and HDAC inhibitors in a variety of tumor models, including HL.80,86 A phase I clinical trial combining the HDAC inhibitor panobinostat with the mTOR inhibitor everolimus is currently enrolling patients with NHL and HL.
NF-κB
NF-κB plays a central role in regulating the expression of various genes involved in cell survival, apoptosis, carcinogenesis, and inflammation, making it a potential therapeutic target.87 The NF-κB family is composed of five proteins: NF-κB1 (p50/p105), NF-B2 (p52/p100), RelA (p65), RelB, and c-Rel. These members exist as homodimers and heterodimers that are organized into two distinctive pathways: the classical (or cononical) and the alternative (non-cononical) pathways. At the center of the classical pathway is the p50/p65 heterodimer. In unstimulated cells, p50/p56 is present in the cytoplasm in an inactive form, bound to inhibitors of NF-κB (IκBα, IκBβ, and IκBε). Upon activation, IκB is rapidly phosphorylated and ubiquitinated and subsequently degraded by the proteasome. Consequently, the active p50/p65 heterodimer is translocated to the nucleus to induce transcription of target genes.88 Activation of the alternative pathway results in the RelB/ p52 and RelB/p50 dimers that also translocate to the nucleous and induce gene transcription. Both pathways have shown to be activated in primary and cultured HRS cells of HL and to be involved in promoting HRS cell survival.4,7,89,–91 In addition to autocrine and paracrine cytokine loops that can activate NF-κB in HRS cells, mutations in the IκB and A20 genes were also reported to be involved in the aberrant activation of NF-κB in HRS cells.4,92,93
The first attempt to therapeutically inhibit NF-κB activation in HL used the proteasome inhibitor bortezomib. By inhibiting the degradation of cytoplasmic IκBα, bortezomib inhibits the activation of NF-κB. Furthermore, bortezomib has been reported to alter the levels of p21, p27, Bcl-2, Bax, XIAP, survivin, and p53, leading to cell cycle arrest and apoptosis in several tumor types.94 In preclinical studies, bortezomib inhibited HL cell line proliferation and induced cell cycle arrest and apoptosis in a time-dependent and dose-dependent manner, and was effective even in HL cell lines that harbored mutations in the IκBα gene.95 Furthermore, bortezomib enhanced the effect of gemcitabine chemotherapy and potentiated the effect of anti-CD30 antibody and TRAIL/APO2L.95,96 Despite these favorable preclinical results, bortezomib demonstrated no significant clinical activity in patients with relapsed HL.97,98
Based on preclinical experiments that demonstrated synergy between bortezomib and chemotherapy, two independent groups evaluated bortezomib-based combinations in patients with relapsed classical HL. In the first study, a phase I trial was conducted to evaluate the combination of bortezomib with the ICE regimen.99 Bortezomib was given at doses 1, 1.3, or 1.5 mg/m2 on days 1 and 4 of each ICE cycle. Twelve patients were enrolled, of whom 6 achieved PRs and 3 achieved CRs, for an overall response rate of 75%. Treatment was well tolerated and was associated with reversible grade 4 neutropenia and thrombocytopenia in 33% and 50% of the patients, respectively. Based on these encouraging data, a randomized phase II study comparing ICE with bortezomib plus ICE is currently enrolling patients to determine the contribution of bortezomib to the ICE regimen. In a second study, bortezomib was combined with gemcitabine for the treatment of patients with relapsed HL.100 Bortezomib 1 mg/ m2 was given on days 1, 4, 8, and 11, and gemcitabine 800 mg/m2 was given on days 1 and 8. Treatments were repeated every 21 days. The overall response rate in 18 patients who were enrolled on study was 22%. Because of the relatively low response rate, coupled with treatment-related liver toxicity, the authors concluded that this regimen should not be further developed for the treatment of HL.
Heat Shock Protein 90 (HSP90)
HSPs are cellular chaperone proteins that are required for essential housekeeping functions such as protein folding, assembly, and transportation across different cell compartments. HSPs also promote cell survival by maintaining the structural and functional integrity of several client proteins that regulate cell survival, proliferation, and apoptosis.101,102 HSP90 is frequently overexpressed in cancer cells. Because HSP90 selectively interacts with and stabilizes several key signaling proteins, protein kinases, and oncogenic signal transduction proteins, it became an attractive target for cancer therapy. Similar to other cancers, HSP90 is overexpressed in primary and cultured HL cells.103,104 HSP90 chaperones several client proteins that are known to promote HRS cell survival, including ERK, Akt, and NF-κB.8,95,105 In a preclinical experiment, the HSP90 small molecule inhibitor 17-AAG downregulated Akt and cFLIP and induced apoptosis in HL-derived cell lines.103 Furthermore, 17-AAG synergized with doxorubicin and agonistic anti-TRAIL death receptors antibodies. Based on this data, a phase II study of 17-AAG is currently enrolling patients with relapsed HL, and the results are expected to be available in the near future.
Targeting the Microenvironment and Other Immunotherapy
HRS cells are surrounded by an overwhelming number of reactive cellular infiltrate that frequently provide survival signals. In fact, once HRS cells are taken out of their microenvironment, it is extremely difficult to grow them in culture. Strategies to disrupt the supportive role of the microenvironment are currently being examined for the treatment of HL. One strategy is to selectively deplete specific cellular compartments by using monoclonal antibodies, such as rituximab. In a different strategy, immunomodulating drugs, such as revlamid, are used to activate T and NK cells to induce a favorable anti-tumor response.106 The role of mTOR and HDAC inhibitors in modulating the cellular components in the microenvironment and angiogenesis may also contribute to their anti-lymphoma activity.
Rituximab
The anti-CD20 monoclonal antibody rituximab was evaluated in the treatment of patients with classical HL. Although CD20 antigen is infrequently expressed by HRS cells, it is highly expressed by the reactive B cells in the microenvironment. Thus, it was hypothesized that rituximab may induce clinical remissions in classical HL by depleting B cells from the microenvironment, by directly killing the few cases of CD20-expressing HRS cells, and perhaps by killing the putative HRS stem cells.107 In a pilot study, investigators from the M.D. Anderson Cancer Center treated 22 patients with relapsed classical HL with six weekly doses of rituximab, of whom 6 demonstrated CD20 expression by HRS cells.108 Five (22%) patients achieved partial or complete remissions, and 8 additional patients had stable disease. Clinical remissions were observed in patients regardless of CD20 expression by HRS cells, and were limited in patients whose disease was confined to the lymph nodes.
In a follow-up study, the same investigators combined rituximab with ABVD (adriamycin, bleomycin, vinblasine, dacarbazine) chemotherapy to treat patients with newly diagnosed classical HL.109 Fifty-two patients with newly diagnosed classical HL were treated on a phase II study. With a median follow-up of 32 months, the estimated event-free survival (EFS) was 82% and overall survival 100%. Importantly, the EFS was improved for all risk categories: for patients with a prognostic score of 0 to 1 the EFS was 92%, for scores 0 to 2, 86%, and for scores 3 to 5, 73%. These data are currently being confirmed in a multicenter randomized study comparing ABVD with rituximab plus ABVD.
Lenalidomide
Two independent groups evaluated the safety and efficacy of lenalidomide in patients with relapsed HL. In the first study, Fehniger et al reported their experience with 25 mg/ day of lenalidomide on days 1–21 of a 28-day cycle.110 Treatment continued until progressive disease or an unacceptable adverse event. Despite the liberal dose reductions that were allowed for hematologic and non-hematologic toxicity, 4 of the 12 evaluable patients responded (1 CR and 3 PRs). Grade 3 and 4 neutropenia was observed in 47%, and thrombocytopenia was seen in 27% of the patients. In a second study, Kuruvilla and colleagues treated 15 patients with relapsed HL using the same dose and schedule of lenalidomide in the previous study.111 Two patients achieved PRs and 7 achieved stable disease, with a median time to progression of 3.2 months. Six patients discontinued therapy because of disease progression and 5 due to toxicity. Four patients developed grade 3 or 4 neutropenia and thrombocytopenia and 5 patients developed skin grade 1 or 2 skin rash. Collectively, these data suggest that lenalidomide has promising single-agent activity in relapsed HL.
Autologous LMP2-specific Cytotoxic T Lymphocytes (CTL) for the Treatment of Relapsed EBV+ HL
HRS cells that are infected with the Epstein-Barr virus (EBV) express several viral antigens that may serve as targets for T-cell therapy.112 Initial clinical studies using polyclonal EBV-specific CTLs have been shown to induce clinical remissions in patients with relapsed EBV+ HL.113 Analyses of EBV-CTL lines showed that small populations of T cells reactive against the tumor-associated antigen LMP2 were present in the majority of the infused lines, with some expansion in the peripheral blood following infusion, suggesting that CTLs specifically targeting LMP2 might have greater efficacy in these patients. In a recent study, LMP2-CTLs were generated from 14 patients with EBV+ lymphomas (HL and NHL).114 Polyclonal LMP2-CTL lines recognized 1 to 7 LMP2 epitopes. Using this approach, 5 out of 6 patients who received LMP2-CTL as adjuvant therapy post–stem cell transplantation or chemotherapy remain in remission up to 22 months post-LMP2-CTL infusion. To improve the efficacy and efficiency of this approach, Bollard and colleagues used gene transfer into antigen-presenting cells (APCs) to augment the expression and immunogenicity of LMP2.115 These modified APCs increased the frequency of LMP2-specific CTLs by up to 100-fold compared with unmodified LCL-APCs. The LMP2-specific population expanded and persisted in vivo without adverse effects. Nine of 10 patients treated in remission of high-risk disease remain in remission, and 5 of 6 patients with active relapsed disease had a tumor response, which was complete in 4 and sustained for more than 9 months. In a different approach, Di Stasi and colleagues hypothesized that for the adoptive transfer of tumor-directed T lymphocytes to be effective, a match between the chemokines the HRS cells produce and the chemokine receptors the effector T cells express will be needed. Taking an advantage of the fact that HRS cells produce thymus- and activation-regulated chemokine/CC chemokine ligand 17 (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22), they transfected effector cells with the TARC receptor CCR4, which enhanced their migration to HRS environment.116 Furthermore, T lymphocytes expressing both CCR4 and a chimeric antigen receptor directed to CD30 sustain their cytotoxic function and cytokine secretion in vitro and produce enhanced tumor control when infused intravenously in mice engrafted with human HL. These proof-of-principle experiments provide very encouraging results for an improved efficacy of T-cell therapy in patients with HL and will soon be examined in clinical trials in patients with relapsed HL.
Summary and Future Directions
After more than three decades of quiet on the drug development front, several compounds have been identified as promising agents for the treatment of patients with relapsed classical HL. The two leading compounds, SGN-35 and panobinostat, are currently being examined in pivotal clinical trials seeking approval by the FDA. If approved, these compounds will rapidly change the standard of care for this disease. For example, SGN-35 may be combined with ABVD to improve the cure rate of patients with poor-risk features. Furthermore, it will possible to investigate whether the addition of SGN-35 to front-line therapy can reduce the number of chemotherapy cycles and/or eliminate the need for radiation therapy in patients with good risk features and those with early-stage disease, and therefore may reduce treatment-related toxicity. Additional strategies may incorporate SGN-35 with salvage regimens and in conjunction with stem cell transplantation. Because of the potential genotoxic effects of HDAC inhibitors, their role in front-line regimens will require more careful examination. However, HDAC inhibitors may have a significant role in combination with other drugs in relapsed settings, as they are known to potentiate the efficacy of a variety of chemo-therapeutic drugs. In the future, it would be important to consider combining several new agents, such as SGN-35 and HDAC inhibitors, to build new non-chemotherapy based regimens that will maintain a high cure rate but will also reduce the treatment-related toxicity. Finally, as more novel drugs are identified, future investigations should focus on identifying predictive markers that will lead to more personalized therapeutic approach.
Summary results of selected novel agents in relapsed Hodgkin lymphoma (HL).
| Agent . | Target . | route . | Phase . | N . | PR . | CR . | PR + CR . | 1st author . |
|---|---|---|---|---|---|---|---|---|
| HDACs indicates histone deacetylases; mTOR, mammalian target of rapamycin. | ||||||||
| SGN3014 | CD30 | IV | I/II | 15 | 0 | 0 | 0 (0%) | Bartlett |
| MDX06015 | CD30 | IV | II | 47 | 2 | 2 | 4 (8%) | Ansell |
| SGN3521 | CD30 | IV | I (q3 weeks) | 44 | 6 | 11 | 17 (39%) | Younes |
| SGN3522 | CD30 | IV | I (weekly) | 17 | 1 | 7 | 8 (47%) | Bartlett |
| MGCD0103117 | HDACs | Oral | II | 21 | 6 | 2 | 8 (38%) | Younes |
| Panobinostat118 | HDACs | Oral | I | 20 | 8 | 0 | 8 (40%) | DeAngelo |
| Panobinostat70 | HDACs | Oral | II | 27 | 4 | 1 | 5 (18%) | Younes |
| Vorinostat71 | HDACs | Oral | II | 25 | 1 | 0 | 1 (4%) | Kirshbaum |
| Lenalidomide110 | ? | Oral | II | 12 | 3 | 1 | 4 (33%) | Fehniger |
| Lenalidomide111 | ? | Oral | II | 15 | 2 | 0 | 2 (13%) | Kuruvilla |
| Everolimus85 | mTOR | Oral | II | 17 | 8 | 1 | 9 (53%) | Johnston |
| Agent . | Target . | route . | Phase . | N . | PR . | CR . | PR + CR . | 1st author . |
|---|---|---|---|---|---|---|---|---|
| HDACs indicates histone deacetylases; mTOR, mammalian target of rapamycin. | ||||||||
| SGN3014 | CD30 | IV | I/II | 15 | 0 | 0 | 0 (0%) | Bartlett |
| MDX06015 | CD30 | IV | II | 47 | 2 | 2 | 4 (8%) | Ansell |
| SGN3521 | CD30 | IV | I (q3 weeks) | 44 | 6 | 11 | 17 (39%) | Younes |
| SGN3522 | CD30 | IV | I (weekly) | 17 | 1 | 7 | 8 (47%) | Bartlett |
| MGCD0103117 | HDACs | Oral | II | 21 | 6 | 2 | 8 (38%) | Younes |
| Panobinostat118 | HDACs | Oral | I | 20 | 8 | 0 | 8 (40%) | DeAngelo |
| Panobinostat70 | HDACs | Oral | II | 27 | 4 | 1 | 5 (18%) | Younes |
| Vorinostat71 | HDACs | Oral | II | 25 | 1 | 0 | 1 (4%) | Kirshbaum |
| Lenalidomide110 | ? | Oral | II | 12 | 3 | 1 | 4 (33%) | Fehniger |
| Lenalidomide111 | ? | Oral | II | 15 | 2 | 0 | 2 (13%) | Kuruvilla |
| Everolimus85 | mTOR | Oral | II | 17 | 8 | 1 | 9 (53%) | Johnston |
Targeted therapy of HRS cells. HRS cells express a variety of receptors and antigens that can be targeted by monoclonal antibodies. Many of these receptors trigger well-defined signaling pathways that promote HRS cell survival. These signaling pathways can be targeted by a variety of small molecules.
Targeted therapy of HRS cells. HRS cells express a variety of receptors and antigens that can be targeted by monoclonal antibodies. Many of these receptors trigger well-defined signaling pathways that promote HRS cell survival. These signaling pathways can be targeted by a variety of small molecules.
Human zinc-dependent histone deacetylases (HDACs) and their inhibitors.
The PI3 kinase/Akt/mTOR pathway is frequently activated in HRS cells. Pharmacologic inhibition of mTOR has produced clinical responses in patients with relapsed classical Hodgkin lymphoma (HL).
The PI3 kinase/Akt/mTOR pathway is frequently activated in HRS cells. Pharmacologic inhibition of mTOR has produced clinical responses in patients with relapsed classical Hodgkin lymphoma (HL).
Disclosures Conflict-of-interest disclosures: The author declares no competing financial interests. Off-label drug use: SGN-35, HDAC inhibitors, Xmab2513.
References
Author notes
Department of Lymphoma/Myeloma, M. D. Anderson Cancer Center, Houston, TX