Abstract
A physician diagnosis of asthma in children and adults with sickle cell disease (SCD) has been associated with increased rates of pain and acute chest syndrome (ACS) episodes and premature death. Despite the clinical significance of a doctor’s diagnosis of asthma in individuals with SCD, the criteria for a physician diagnosis of asthma are not well defined. Many features of asthma are common in individuals with SCD, including symptoms of wheezing, obstructive lung disease and airway hyper-responsiveness. However, it is not clear if these signs and symptoms of asthma reflect a physician diagnosis of asthma, or if these asthma features are related to SCD. Further complicating the diagnosis of asthma in children with SCD is the significant overlap in clinical manifestations between an asthma exacerbation and an ACS episode. Evidence supporting the concept that asthma and SCD are separate co-morbid conditions includes a similar prevalence of asthma between children with SCD and those in the general population and the observation that asthma is inherited in a familial pattern in the families of children with SCD. In contrast, there is significant evidence that asthma-like features may be associated with SCD without a diagnosis of asthma, including a higher than expected prevalence of airway hyper-responsiveness and obstructive lung disease. Regardless of whether SCD and asthma are distinct or overlapping co-morbid conditions, we recommend a systematic and complete evaluation of asthma when the diagnosis is suspected or when patients have multiple episodes of pain or ACS.
Sickle cell disease (SCD) is one of the most common genetic diseases in the United States, occurring in 1 in 2400 births.1 Among African Americans, SCD affects approximately 1 in 400 births and there are about 100,000 individuals in the United States with SCD.2,3 Asthma also disproportionately affects African Americans and is present in about 20% of children.4 In children with SCD, estimates of asthma prevalence are similar to that in children of African descent in the general population,5,6 making asthma one of the most common co-morbid conditions in SCD.
A physician diagnosis of asthma in individuals with SCD is associated with increased SCD-related morbidity and mortality.7–12 Recognizing, appropriately diagnosing and treating asthma in children and adults with SCD may decrease the rate of complications; however, the mechanism by which asthma may influence SCD morbidity is not well defined. To date, all studies demonstrating that asthma is associated with increased SCD severity are based on a doctor diagnosis. The objective criteria used to make a physician diagnosis of asthma are not well defined and may vary from one physician to another. Whether a physician diagnosis of asthma in children with SCD has the same constellation of clinical features that are recognized among children without SCD is not known. Potentially, signs and symptoms suggestive of asthma, such as wheezing or an obstructive pattern on pulmonary function testing, may be related to pulmonary manifestations of SCD and thus represent a different pathophysiology than asthma.
In this review, we will examine the evidence that a physician diagnosis of asthma is associated with an increased rate of pain, acute chest syndrome (ACS) and death. Thereafter, we will review the data for and against the premise that asthma and SCD are distinct co-morbid conditions. Finally, in the absence of evidence-based guidelines, we will discuss our recommendations for the evaluation and therapy of asthma in individuals with SCD.
Asthma Is Associated with Increased Rate of Acute Chest Syndrome
There is significant clinical overlap between ACS and asthma. ACS is variably defined, but the definition typically includes a fever, increased respiratory effort and a new radiodensity on chest radiograph. Other clinical features of ACS include wheezing, chills and cough.13 For children with SCD, almost any admission to the hospital for respiratory syncytial virus bronchiolitis or an asthma exacerbation could be considered a diagnosis of ACS. Given the shared clinical characteristics between asthma and ACS, determining a cause and effect relationship has been a significant challenge.
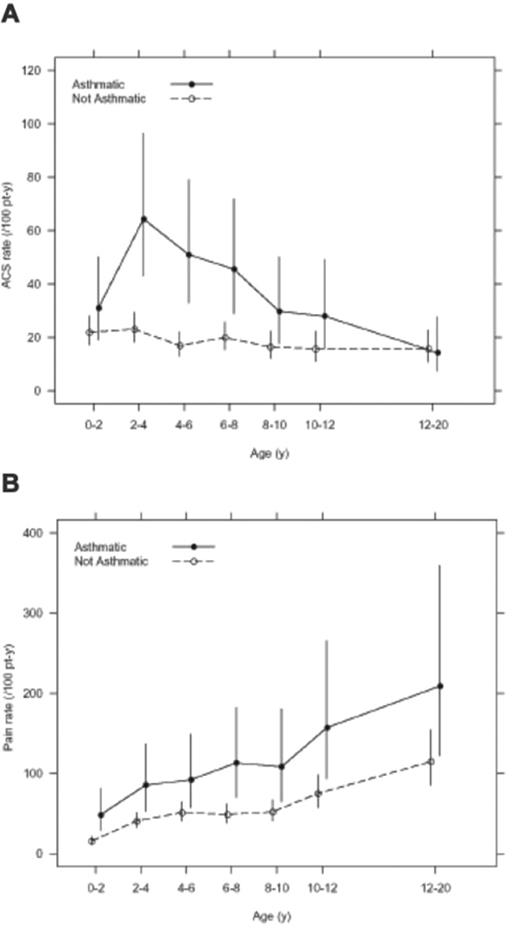
Perin et al provided the initial observation of a relationship between asthma and pain. In 1983, they described a 6-year-old girl with SCD and severe asthma who was admitted to the hospital for an asthma exacerbation and subsequently developed a pain episode.14 Since this original description, several studies have documented the strength of this association.5–12 The largest and most rigorously conducted study was based on the Cooperative Study of Sickle Cell Disease (CSSCD). A prospective cohort of 291 infants with hemoglobin SS were followed for a mean length of 11.0 years with a total follow-up of 4062 patient-years. Forty-nine children in the cohort (16.8%) had asthma; these children had almost a two times greater rate of ACS episodes when compared with children without asthma (0.39 episodes per patient-year versus 0.20 episodes per patient-year, P < .001)11 (Figure 1 ). Taken together, these data indicate that multiple episodes of ACS in a child with SCD should prompt consideration for a diagnosis of asthma.
Children with asthma are also at an increased risk for developing ACS when admitted to the hospital for a pain episode. Boyd et al demonstrated that children with a physician diagnosis of asthma were 4.0 times (95% CI, 1.7–9.5) more likely to develop ACS during the admission than patients without asthma.5 Additionally, Glassberg et al demonstrated in a case-control study that among the children with pain and asthma, the odds ratio of having antecedent or concurrent respiratory symptoms within 96 hours of a pain event was 4.9 (95% CI, 2.2–10.7) when compared with children with pain and without asthma.15 Lastly, in another case-control study, Sylvester et al demonstrated that there was a higher proportion of children who had an ACS episode that were taking asthma medications when compared with those who were not (P = .02).16 Thus, a diagnosis of asthma is not only associated with an increased overall rate of ACS among children with SCD, but is also associated with an increased rate of ACS following admission to the hospital for pain and an increased rate of antecedent respiratory symptoms within 96 hours prior to presenting with pain. The preponderance of evidence for the relationship between asthma and ACS is in children with HbSS; however, some studies included children with milder SCD phenotypes (HbSC, HbSβ-thalassemia+).8,9,15 Given the significant clinical overlap between an asthma exacerbation and an ACS episode, the consistent association between asthma and ACS episodes with different study designs (case-control and cohort studies) and different patient populations (US, England and France) is expected.
The relationship between asthma and ACS in adults with SCD has not been well documented. There are several reasons why there may be no association between these two conditions. First, asthma symptoms often improve as individuals enter their second and third decade of life and airway size increases.17 Second, many adults who were diagnosed with asthma as children are now asymptomatic and do not recall their diagnosis.18 Finally, the incidence of ACS is lower in adults; thus, a relationship between asthma and ACS may be more difficult to assess if one truly exists.19 More rigorous studies either confirming or refuting the association between asthma and a possible increased rate of ACS are needed in adults with SCD.
Asthma Is Associated with Increased Rate of Pain Episodes
Studies examining the relationship between asthma and rate of pain episodes in children with SCD have had inconsistent results, with some studies showing a positive association and other studies revealing no association. The study with the longest follow-up period examining the effect of asthma on pain episodes is from the CSSCD cohort with over 4000 patient-years. In this group of children with rigorously documented pain episodes, a diagnosis of asthma was associated with an increased rate of pain events when compared with children without asthma11 (Figure 1 ). However, another retrospective cohort study that included a large pediatric study from France did not validate the association between asthma and increased rate of pain events. In 297 children with SCD and a total of 1805 patient-years of follow-up, there was not an increased rate of pain among children with asthma6; however, only 25 patients had a diagnosis of asthma. The inconsistent finding between the association of pain and a physician diagnosis of asthma has several explanations, including different definitions of pain that were used in the two studies, physician contact that required an opioid administration (CSSCD) versus hospitalization for pain (France), as well as the small number of patients with asthma in the France cohort. Nevertheless, the association between asthma and pain episodes should be considered preliminary until further evidence documents the association.
Asthma Is Associated with Death in Sickle Cell Disease
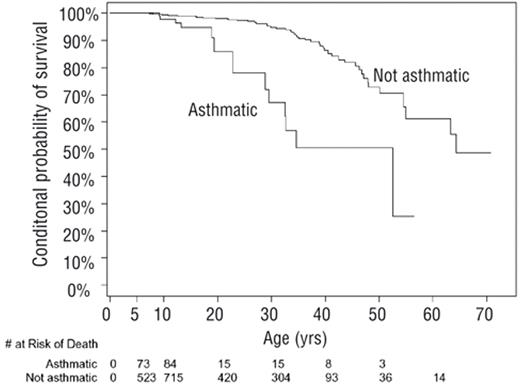
In one study, asthma was found to be associated with premature death in individuals with SCD.10 Examining a group of 1963 children and adults with HbSS followed for approximately 10 years and a total of 18,495 patient-years, a diagnosis of asthma was associated with a two-times increased rate of death (HR 2.36, P = .01), after adjusting for known risks of mortality in individuals with SCD (Figure 2 ). The mean life expectancy for individuals with SCD and asthma was 52.5 years compared with 64.3 years for those without a diagnosis of asthma. The cause of death for these individuals with asthma was not documented. Elevated tricuspid regurgitant jet (TRJ) velocity is also associated with an increased risk of death in adults with SCD20; however, current evidence does not support a relationship between asthma and an elevated TRJ velocity.20–22
Effect of Asthma on Sickle Cell Disease May be Due to Inflammation, V/Q Mismatch, or Both
While there is significant evidence to suggest that asthma modifies SCD severity, the clinical basis for a diagnosis of asthma in children and adults with SCD is not well defined. Many children and adults with SCD do not undergo a rigorous evaluation for asthma, including a thorough personal and family history for asthma symptoms, pulmonary function testing and possibly skin and bronchoprovocation testing. The diagnosis of asthma may be based solely on wheezing symptoms, and thus we can only postulate about the underlying mechanism of asthma in children and adults with SCD. Asthma and SCD are both inflammatory diseases and markers of inflammation are known to be related to SCD severity.19 The inflammation associated with asthma is largely confined to the airways; however, if airway inflammation potentiates vascular inflammation or vice versa, the result may be increased vaso-occlusion and a higher rate of pain and ACS events. Alternatively, ventilation-perfusion (V/Q) mismatch may increase the rate of pain or ACS events in individuals with SCD as was described by Glassberg et al.15 Children may present with a viral syndrome or an asthma exacerbation triggering regional hypoxia, sickling and ultimately systemic vaso-occlusion. These potential etiologies are not mutually exclusive and both may be contributing to the association between asthma and increased SCD-related morbidity and mortality.
Family History of Asthma Is Associated with Increased Pain and ACS
Preliminary evidence suggesting another causal pathway between asthma and SCD is based on the association between a family history of asthma and an increased rate of pain. We postulated that inflammatory genes involved in asthma pathogenesis would predispose to an increased rate of pain episodes even among individuals without a diagnosis of asthma. In a cohort of 211 children with HbSS, the rate of pain and ACS was found to be increased roughly twofold compared with children without a family history of asthma.23 After adjusting for a personal history of asthma, there was still an independent association between a sibling history of asthma and an increased rate of pain. As these children did not have a personal history of asthma, these data suggest that inflammatory genes and their corresponding mediators of asthma pathogenesis may contribute to vascular inflammation.
Leukotrienes Are Inflammatory Mediators that are Elevated and Baseline Levels Are Associated with Pain Events
Inflammatory pathways that are implicated in the pathogenesis of asthma and pain provide additional evidence suggesting that there is a common mechanism between asthma and SCD. Leukotrienes are lipid mediators of inflammation derived from arachidonic acid. Phopholipase A2 liberates arachidonic acid from cell membrane, ultimately leading to the production of leukotriene A4. From LTA4, two classes of leukotrienes are generated, leukotriene B4 and cysteinyl leukotrienes (CysLTs).24 LTB4 primarily promotes neutrophil activation and chemotaxis, while CysLTs have actions in the lung and the vasculature. In the lung, effects of CysLTs include bronchoconstriction,25 smooth muscle proliferation,26 mucus production27 and airway edema,28 while in the vasculature CysLTs promote vasoconstriction,29 vascular leakage and up-regulation of cellular adhesion molecules.30 CysLTs are important to asthma pathogenesis, and levels of CysLTs correlate with measures of asthma severity,31 asthma exacerbations32 and presence of exercise-induced asthma.33
LTB4 and CysLT levels are increased in individuals with SCD when they are well and during vaso-occlusive events. Although LTB4 levels have a lesser role in the process of asthma, its actions on the activation, migration and adhesion of neutrophils to endothelium suggest that LTB4 could contribute to the process of vaso-occlusion. In individuals with SCD, levels of LTB4 are increased compared with controls and, during a pain or ACS episode, the levels increase further from baseline.34
There is stronger evidence implicating the role of CystLTs levels being associated with the pathogenesis of pain. In two separate studies of children and adults with SCD, baseline levels of CysLTs were significantly elevated in those with SCD compared with controls.35,36 Among children with SCD and asthma, CysLT levels were higher than in children with SCD but without asthma. When CysLT levels were measured during pain episodes, there was a significant increase from baseline levels within the same individual.37 Although elevated levels of CysLTs may be related to an underlying diagnosis of asthma in some cases, levels were also elevated in individuals without a history of asthma. Taken together, these data suggest that leukotrienes may contribute to the process of vaso-occlusion.
The mechanism as to why baseline CysLT levels are elevated in children and adults with SCD has not been fully elucidated. There is evidence to suggest that the leukotriene pathway is upregulated through increased phospholipase A2 and 5-lipooxygenase activating protein (FLAP). Levels of circulating secretory phospholipase A2 are increased in many individuals with SCD, and elevated levels have been associated with development of ACS episodes38,39; however, it is not clear how closely secretory phospholipase A2 in the plasma correlates with intracellular levels, the site of action for leukotriene generation. One potential mechanism for an increase in CysLT was demonstrated by Patel et al when they provided evidence that transcription of FLAP was increased in peripheral blood mononuclear cells in individuals with SCD and that activation of FLAP was potentially mediated through placenta growth factor.40 Dissecting the leukotriene pathway to determine the underlying pathogenesis of leukotriene elevation in individuals with SCD may reveal new therapeutic targets for the treatment or prevention of pain.
Potential Role of Nitric Oxide in Sickle Cell Disease and Asthma
Several studies have demonstrated that NO is a mediator of SCD-related morbidity and mortality.20,41 Among individuals with SCD, hemolysis with release of cell free hemoglobin and arginase reduces NO bioavailability in the vasculature.42 Reduced NO bioavailability is related to an elevated TRJ velocity, which is associated with an increased risk of death.20 Individuals with increased hemolysis and correspondingly reduced NO bioavailibility also have an increased incidence of priapism and leg ulcers, but fewer pain and ACS episodes.42,43 This observation has led to the proposed classification of individuals with SCD into either the hemolytic phenotype characterized by an elevated TRJ velocity, priapism and leg ulcers, or the viscosity-vaso-occlusive phenotype characterized by frequent pain and ACS episodes and an increased incidence of avascular necrosis.42 The NO pathway in airway lining fluid and epithelium has also been implicated in asthma pathogenesis.44 Similar to patients with SCD, individuals with asthma have increased arginase activity and decreased levels of arginine in the plasma.45 Despite the critical role of altered NO homeostasis in the pathogenesis of asthma and SCD, a diagnosis of asthma has not been related to increased TRJ velocity in several studies.20–22 Elevated TRJ velocity has been associated with pulmonary function; however, the evidence demonstrates a relationship between TRJ velocity and low forced vital capacity, consistent with mild restrictive defects.21,46 No studies to date have linked obstructive lung disease to hemolysis or an elevated TRJ velocity. Taken together, these data provide little evidence implicating the NO pathway in the pathogenesis of asthma in children and adults with SCD.
Exhaled NO is a marker of asthma severity that has been examined in individuals with SCD. Elevated exhaled NO is suggestive of an asthma diagnosis and predicts response to oral or inhaled corticosteroids.47 It is not clear if exhaled NO contributes to an asthma phenotype or if exhaled NO is just a marker for asthma. Among individuals with SCD, several studies have reported decreased levels of exhaled NO, although no study has specifically evaluated individuals with SCD and asthma. Lower levels of exhaled NO have been associated with ongoing pain episodes,48 prior episodes of ACS49 and chronic lung disease.50 The role of exhaled NO in the diagnosis or management of asthma in individuals with SCD has not been evaluated, although prior data would suggest that pre-existing lung disease and/ or reduced plasma NO may limit the utility of exhaled NO in this patient population. Given the paucity of evidence implicating the NO pathway in the process of asthma in individuals with SCD, no definitive conclusions can be made; however, future studies of asthma in this patient population should examine the relationship between hemolysis and features of asthma.
Evidence to Support Asthma and Sickle Cell Disease as Distinct, Co-Morbid Diseases
The concept that asthma and SCD are distinct conditions is supported by two lines of evidence (Table 1 ). First, the prevalence of asthma in children with SCD is similar to reports in urban African-American children. For reasons not well understood, asthma disproportionately affects African-American children in the United States with a prevalence of about 20%.4 In a case-control study of 139 children with HbSS designed to examine the relationship between asthma and ACS, the prevalence of asthma was 12% in children without evidence of ACS.5 Other studies have also reported a prevalence of asthma in children with SCD that is comparable to the general population.6 Another independent line of evidence is that asthma shows a familial pattern of inheritance in the families of children with SCD.51 In a study of 104 families identified from affected children with SCD, 20% of parents and 32% of siblings reported a diagnosis of asthma. Statistical modeling of the inheritance pattern of asthma among first-degree relatives of children with SCD and asthma demonstrate a significant major gene effect for the transmission of asthma in these families.
Evidence to Suggest that Asthma Symptoms Are a Manifestation of Sickle Cell Disease
Airway hyper-responsiveness and pulmonary function abnormalities are more common in children and adults with SCD than in the general population, suggesting that these clinical features of asthma may be related to the pathogenesis of SCD52–58 (Table 1 ). Measures of airway hyper-responsiveness are thought to be relatively specific for the diagnosis of asthma in children and adults in the general population.57 The degree of airway hyper-responsiveness to a stimulus, most commonly the parasympathetic agonist methacholine, reflects airway inflammation and provides evidence for a diagnosis of asthma. The prevalence of airway hyper-responsiveness in children and adults with SCD has been reported as high as 78%56; in contrast, the prevalence of airway hyper-responsiveness is 18% in children in the general population.60 Further, the presence or absence of airway hyper-responsiveness has not been clearly associated with a diagnosis of asthma in children or adults with SCD.54 Presumably, the significant number of individuals with SCD and airway hyper-responsiveness reflects non-specific airway inflammation that may be related to the process of SCD. Inflammation is known to be critical to the pathogenesis of pulmonary complications in SCD, and anti-inflammatory therapies have demonstrated efficacy in the treatment of ACS.61,62 Although ACS likely represents a heightened state of airway inflammation, low-grade inflammation in the lungs may be present in many individuals with SCD at baseline.
Obstructive pulmonary function patterns are also common in individuals with SCD and not clearly explained by an asthma diagnosis. Among children with SCD, 35% demonstrate obstructive defects on pulmonary function testing.57 Rates of pain and ACS episodes have been associated with obstructive lung disease in children with SCD, although the relationship has been inconsistent.58,63,64 Despite conflicting data, the preponderance of evidence suggests that there is a relationship between SCD severity and pulmonary function abnormalities; however, data to suggest that asthma has a significant role in the many of cases of obstructive lung disease in individuals with SCD are limited.
Summary and Recommendations
Significant clinical evidence demonstrates that a physician diagnosis of asthma increases SCD severity. However, the clinical and laboratory features that lead physicians to diagnose a child or adult with asthma are not well defined. Extrapolating the prevalence of asthma in the general population to children with SCD, we would expect that a significant portion of children with SCD will also have asthma. Data to suggest that children with SCD and asthma will or will not present with the same clinical features that are seen in children without SCD are limited. Pulmonary complications are common in individuals with SCD, and thus the interaction between asthma, ACS, pulmonary function abnormalities and SCD-related morbidity and mortality is likely complex and overlapping. Different etiologies, some related to SCD and others related to asthma, may lead to an asthma diagnosis. Distinguishing a diagnosis of asthma that may be responsive to oral or inhaled corticosteroids from wheezing related to ACS, pneumonia, or baseline lung function abnormalities is required to treat individuals appropriately and potentially dampen the severity of SCD.
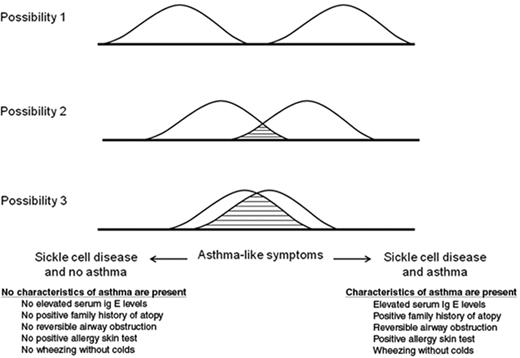
There are three possibilities to describe the relationship between asthma and SCD, ranging from asthma and SCD as distinct, non-overlapping diseases to asthma symptoms largely being related to SCD pathogenesis (Figure 3 ). Based on the significant overlap between the clinical presentation of asthma and ACS, we postulate there are likely some individuals with SCD who have symptoms of wheezing and are diagnosed with asthma, but have few other characteristics of asthma. The diagnosis of asthma in these individuals may be related to SCD. In contrast, asthma is present in a large number of African-American children and it must be present in some children with SCD also.
In the absence of established guidelines for the evaluation and management of asthma in individuals with SCD, we recommend rigorous testing for all children and adults with SCD that mirrors the evaluation for asthma in the general population (Table 2 ). Despite the paucity of evidence that is available to guide clinical practice, treating children and adults with SCD who have signs or symptoms of asthma aggressively with asthma therapies, similar to any individual with a diagnosis of asthma, is likely the best approach to dampen the impact of asthma on SCD until further studies are completed.
Evidence for and against asthma as a distinct co-morbid disease in individuals with sickle cell disease (SCD).
| Pros . | References . | Cons . | References . |
|---|---|---|---|
| Similar prevalence of asthma in the general population and in children with SCD | 4–6 | Higher prevalence of airway hyper-responsiveness in individuals with SCD when compared with the general population | 52–56 |
| Familial pattern of asthma inheritance among children with SCD | 51 | Higher prevalence of airway obstruction in children with SCD when compared with the general population | 57 |
| Overlap of symptoms between acute chest syndrome and asthma | 13 |
| Pros . | References . | Cons . | References . |
|---|---|---|---|
| Similar prevalence of asthma in the general population and in children with SCD | 4–6 | Higher prevalence of airway hyper-responsiveness in individuals with SCD when compared with the general population | 52–56 |
| Familial pattern of asthma inheritance among children with SCD | 51 | Higher prevalence of airway obstruction in children with SCD when compared with the general population | 57 |
| Overlap of symptoms between acute chest syndrome and asthma | 13 |
Recommended asthma evaluation in children and adults with sickle cell disease.
| A. Clinical Assessment . | |||
|---|---|---|---|
| Test . | Frequency . | Rationale . | References . |
| Review of systems for atopy, asthma | Annually, starting at one year of age If history is positive, refer to a pulmonologist | Individuals with asthma and SCD are at increased risk of vaso-occlusive episodes, acute chest syndrome and death | 7–12 |
| Assessment of lung function by spirometry and lung volumes by plethysmography | For children, starting at 6 years of age: every 5 years in children with no asthma or ACS episodes and every 2–3 years in children with asthma or ACS | Children with SCD may have obstructive defects that predispose to increased SCD morbidity | 57,64 |
| For adults, at least once and every 2–3 years if abnormal If there is evidence of obstruction or restriction, refer to pulmonologist | Adults with SCD have a high incidence of restrictive defects | 58 | |
| B. Treatment | |||
| Therapy | Frequency | Rationale | References |
| Treatment of individuals with SCD and asthma per NHLBI guidelines | Indefinitely | Treatment of persistent asthma with daily inhaled corticosteroids is effective in reducing asthma hospitalizations and symptom days | NHLBI guidelines 2007 |
| Appointment with pulmonologist | At least annually for individuals with SCD and mild asthma At least every 6 months for individuals with SCD and moderate to severe asthma | Individuals with asthma and SCD are at increased risk of vaso-occlusive episodes, acute chest syndrome and death | 7–12 |
| A. Clinical Assessment . | |||
|---|---|---|---|
| Test . | Frequency . | Rationale . | References . |
| Review of systems for atopy, asthma | Annually, starting at one year of age If history is positive, refer to a pulmonologist | Individuals with asthma and SCD are at increased risk of vaso-occlusive episodes, acute chest syndrome and death | 7–12 |
| Assessment of lung function by spirometry and lung volumes by plethysmography | For children, starting at 6 years of age: every 5 years in children with no asthma or ACS episodes and every 2–3 years in children with asthma or ACS | Children with SCD may have obstructive defects that predispose to increased SCD morbidity | 57,64 |
| For adults, at least once and every 2–3 years if abnormal If there is evidence of obstruction or restriction, refer to pulmonologist | Adults with SCD have a high incidence of restrictive defects | 58 | |
| B. Treatment | |||
| Therapy | Frequency | Rationale | References |
| Treatment of individuals with SCD and asthma per NHLBI guidelines | Indefinitely | Treatment of persistent asthma with daily inhaled corticosteroids is effective in reducing asthma hospitalizations and symptom days | NHLBI guidelines 2007 |
| Appointment with pulmonologist | At least annually for individuals with SCD and mild asthma At least every 6 months for individuals with SCD and moderate to severe asthma | Individuals with asthma and SCD are at increased risk of vaso-occlusive episodes, acute chest syndrome and death | 7–12 |
Age-specific incidence of acute chest syndrome episodes (panel A) and pain episodes (panel B) in the infant sickle cell anemia cohort from the Cooperative Study of Sickle Cell Disease. Reprinted with permission from Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927.11
Age-specific incidence of acute chest syndrome episodes (panel A) and pain episodes (panel B) in the infant sickle cell anemia cohort from the Cooperative Study of Sickle Cell Disease. Reprinted with permission from Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108:2923–2927.11
Kaplan-Meier plot of age of death for subjects with sickle cell anemia and asthma (n = 138) and those without asthma (n = 25), conditional on survival beyond age 5 years. Reprinted with permission from Boyd JH et al. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–1118.10
Kaplan-Meier plot of age of death for subjects with sickle cell anemia and asthma (n = 138) and those without asthma (n = 25), conditional on survival beyond age 5 years. Reprinted with permission from Boyd JH et al. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–1118.10
Three possible descriptions of the relationship between sickle cell disease (SCD) and asthma. Possibility 1 shows SCD and asthma as distinct diseases. Possibility 2 depicts SCD and asthma as distinct diseases with some overlapping asthma symptoms and characteristics. Possibility 3 describes the scenario whereby asthma symptoms are related almost entirely to the pathogenesis of SCD.
Three possible descriptions of the relationship between sickle cell disease (SCD) and asthma. Possibility 1 shows SCD and asthma as distinct diseases. Possibility 2 depicts SCD and asthma as distinct diseases with some overlapping asthma symptoms and characteristics. Possibility 3 describes the scenario whereby asthma symptoms are related almost entirely to the pathogenesis of SCD.
Disclosures Conflict-of-interest disclosure: JJF receives research funding from TRF Pharma. MRD declares no competing financial interests. Off-label drug use: None disclosed.
Acknowledgments
Supported in part by the National Institutes of Health, National Heart, Lung and Blood Institute, RO1 HL079937 (MRD), K12HL08710 (JJF), and Burroughs Wellcome Foundation (MRD).
References
Author notes
Department of Medicine, Division of Hematology, Washington University School of Medicine, St. Louis, MO
Department of Pediatrics, Division of Genetics, Washington University School of Medicine, St. Louis, MO