Abstract
Although drugs are a common cause of acute immune-mediated thrombocytopenia in adults, the drug etiology is often initially unrecognized. Most cases of drug-induced thrombocytopenia (DITP) are caused by drug-dependent antibodies that are specific for the drug structure and bind tightly to platelets by their Fab regions but only in the presence of the drug. A comprehensive database of 1301 published reports describing 317 drugs, available at www.ouhsc.edu/platelets, provides information on the level of evidence for a causal relation to thrombocytopenia. Typically, DITP occurs 1 to 2 weeks after beginning a new drug or suddenly after a single dose when a drug has previously been taken intermittently. However, severe thrombocytopenia can occur immediately after the first administration of antithrombotic agents that block fibrinogen binding to platelet GP IIb-IIIa, such as abciximab, tirofiban, and eptifibatide. Recovery from DITP usually begins within 1 to 2 days of stopping the drug and is typically complete within a week. Drug-dependent antibodies can persist for many years; therefore, it is important that the drug etiology be confirmed and the drug be avoided thereafter.
Drug-induced thrombocytopenia (DITP), which also includes thrombocytopenia induced by beverages, foods, and herbal remedies, is an important clinical problem for hematologists. DITP typically appears suddenly, is often severe, and can cause major bleeding and death.1–3 In many patients, the drug etiology is not initially recognized. In hospitalized patients, unexpected thromb-ocytopenia may be attributed to complications such as sepsis. In previously asymptomatic patients, DITP is often misdiagnosed as immune thrombocytopenic purpura (ITP) with resulting inappropriate treatment. Even when the diagnosis of DITP is considered, a drug etiology may not be apparent because patients may not think that self-regulated medications, beverages, foods, or herbal remedies are relevant to their bleeding symptoms and therefore they may not report them to their physician.4–8
In this review, we first describe the current understanding of the pathogenesis of DITP. Next we address a series of questions concerning the sequence of evaluation and management of patients with suspected DITP:
When should DITP be suspected?
Which among the multiple drugs (or beverages, foods, or herbal remedies) that the patient is taking may be responsible for thrombocytopenia?
How can a drug etiology be confirmed?
What is the clinical course and appropriate management of DITP?
What is the physician’s responsibility for reporting the association of the drug with thrombocytopenia?
The focus of this review will be on immune-mediated DITP caused by peripheral platelet destruction, rather than thrombocytopenia caused by dose-dependent marrow suppression. This review will also focus only on isolated thrombocytopenia; reports of systemic disorders that include thrombocytopenia, such as quinine-induced thrombotic thrombocytopenic purpura-hemolytic uremic syndrome,9,10 are not discussed. Heparin-induced thromb-ocytopenia will not be considered since its pathogenesis, clinical course, and management are distinct from other immune-mediated drug-induced thrombocytopenias and because thrombosis, not thrombocytopenia, is the major adverse complication.
What Is the Pathogenesis of the Drug-induced Thrombocytopenia?
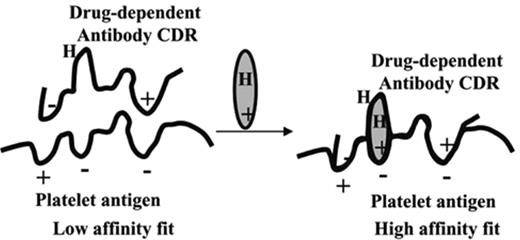
DITP is an idiosyncratic immune-mediated reaction. Drug-dependent antibodies are an unusual class of antibodies that bind firmly to specific epitopes on platelet surface glycoproteins only in the presence of the sensitizing drug.2,3,11 Drug-dependent antibodies are very specific for the drug structure. These antibodies may be derived from a naturally occurring pool of immunoglobulins that are weakly reactive autoantibodies with affinities for epitopes on platelet membrane glycoproteins that are insufficient to cause binding in the normal circulation.11,12 It has been proposed that the sensitizing drugs typically contain charged and/or hydrophobic structural elements that enable them to bind to both the antibody and platelet surface proteins. In this model, the drugs bind noncovalently and reversibly to platelets, commonly to sites on GP IIb-IIIa and/or GP Ib-V-IX, and also to the antibody. The resulting “sandwich” facilitates formation of a tight bond between the antibody and the platelet epitope (Figure 1 ). Antibodies induced upon exposure to the sensitizing drug are selected by the ability of their Fab domains to recognize the drug bound to the platelet epitope. Drug-dependent antiplatelet antibodies typically occur after exposure to a new drug for 1 to 2 weeks. Drug-dependent antibodies can also occur following intermittent use of a drug for a long time. For unknown reasons, platelets are the target for drug-dependent antibodies much more often than neutrophils or red cells.
The acute immune-mediated thrombocytopenia caused by the antithrombotic agents that block fibrinogen binding to GP IIb-IIIa has distinct clinical characteristics; 0.1% to 2.0% of patients may have severe thrombocytopenia within several hours of their first exposure to abciximab, tirofiban or eptifibatide, and as many as 12% of patients may become acutely thrombocytopenic after a second exposure to abciximab.2,3 The immediate reactions are the result of naturally occurring antibodies that recognize the murine structural elements of abciximab or structural changes in GP IIb-IIIa caused by binding of tirofiban or eptifibatide. Eptifibatide-dependent anti-platelet antibodies can also induce platelet activation by binding to the platelet FcγRIIa receptor.13 This is reminiscent of heparin-dependent antibodies and could explain paradoxical thrombosis seen in some patients with thrombocytopenia induced by these agents.13 In some patients, the onset of thrombocytopenia is delayed for up to a week after abciximab administration, when abciximab-dependent antibodies are induced at a time when abciximab remains in the circulation.14
The common pathogenetic feature among all drugs that cause immune-mediated thrombocytopenia is that thrombocytopenia only occurs in the presence of the drug. This predicts that thrombocytopenia will begin to resolve within several days of discontinuing the drug and its clearance from the circulation, and also explains why patients may have a high titer of drug-dependent anti-platelet antibodies for many years with no problems—until drug exposure occurs again.
When Should DITP Be Suspected?
The unexpected occurrence of severe thrombocytopenia should always trigger concern for DITP. Patients who have recurrent episodes of thrombocytopenia with prompt recovery should be assumed to have thrombocytopenia caused by a drug or possibly by a beverage, such as the quinine-containing beverages tonic water and bitter lemon,15 food, such as tahini (pulped sesame seeds)4 and Lupinus termis beans,16 or herbal remedies, such as Jui herbal tea.5,6 An example of the risk of misdiagnosis of DITP as ITP was apparent in a cohort of 343 patients registered as ITP;17 28 (8%) of these patients were subsequently diagnosed with DITP. Quinine, including quinine in tonic water, was the most common etiology, occurring in 13 (46%) of the 28 patients. Three of the 28 patients had had a splenectomy before DITP was recognized; splenectomy had no apparent effect on the recurrence of thrombocytopenia with subsequent drug. Detection of quinine and other common remedies that patients take only intermittently and without physician direction requires repeated, explicit questions.7,8
Which of the Multiple Drugs (or Beverages, Foods, or Herbal Remedies) that the Patient Is Taking May Be Responsible for Thrombocytopenia?
Multiple lists from many sources describe thrombocytopenia as a risk for almost all drugs. To better understand the relative risks of the many drugs apparently associated with thrombocytopenia, we have systematically reviewed all published reports of DITP, first in 19981 and subsequently every two years; our most recent review was completed in October 2008. The data are available at www.ouhsc.edu/platelets. Each article is assessed independently by two or more reviewers according to the criteria presented in Table 1 , to establish the level of evidence supporting the reported drug as a cause of thrombocytopenia.1 Among the four criteria for evaluation of individual patient data (Table 1 ), the most difficult criterion to assess in many reports is the documentation that the suspected drug was the only drug used before the onset of thrombocytopenia or that other drugs were continued or re-introduced after discontinuation of the suspected drug, with a sustained normal platelet count. Also it is often difficult to exclude other potential causes of thrombocytopenia, especially in hospitalized patients. Although re-administration of a suspected drug may not be appropriate, it can be important if the drug is commonly accessible in different preparations and future inadvertent exposure is likely (for example, acetaminophen). Often re-administration has already occurred because the patient has taken the suspected drug on multiple occasions and has had recurrent purpura.7;8
The database contains information on 317 drugs in 1185 individual patient reports and an additional 116 articles describing group data. Combining these data, 451 (35%) reports described definite or probable evidence for a causal relation of the suspected drug to thrombocytopenia; 446 (34%) described only possible or unlikely evidence; 407 (31%) were excluded from the review because the data were not evaluable. Most exclusions were for insufficient information (48%).
There are important limitations to this database: (1) We assume that most occurrences of DITP are not reported in publications. (2) Many articles reported only limited data that were insufficient to provide probable or definite evidence of a causal association of the drug with thrombocytopenia. (3) Articles describing chemotherapeutic drugs were excluded, but at least one chemotherapeutic drug, oxaliplatin, can cause acute, immune-mediated thrombocytopenia.18 (An exception was made and oxaliplatin was added to the database.) (4) Only therapeutic drugs were reviewed;1 foods4 and herbal remedies5,6 that can cause immune-mediated thrombocytopenia on rare occasions are not included. (5) Children are not included since the clinical course of ITP in children, with possible prompt resolution, may be similar to DITP. However, some articles that described children reported definite evidence for a causal relation of the drug to thrombocytopenia.
For our analysis of these data, we have accepted a single report with definite evidence or two or more reports with probable evidence as clinical evidence supporting a causal relation of a drug to thrombocytopenia. We interpreted the presence of two reports with probable evidence as comparable to a re-challenge with the drug in a single report. Only 93 (29%) of the total 317 drugs met these criteria; 21 drugs had 5 or more reports with definite or probable evidence (Table 2 ). However, some of these 21 drugs, such as quinidine and gold, are now rarely used.
The risk of thrombocytopenia with any individual drug is rare. A case-controlled study has estimated the risk of acute thrombocytopenia with trimethoprim/sulfamethoxazole and quinine, the two drugs documented in this study to be the most common causes of thrombocytopenia, to be 38 and 26 cases/106 users/week, respectively.19 Even though quinine and trimethoprim/sulfamethoxazole are also the most commonly reported drugs causing thrombocytopenia among currently used drugs (Table 2 ), the frequency of published reports cannot accurately estimate the frequency of DITP. In a preliminary analysis, we compared the data from published reports to data mining of the FDA Adverse Event Reporting System (AERS), based on reports to the FDA’s MedWatch program, and reports of drug-dependent anti-platelet antibodies detected in samples submitted to the BloodCenter of Wisconsin.20 There was little overlap among the drugs detected by each of the three methods. Systematic analysis of published reports and detection of drug-dependent antibodies may provide specific but incomplete data. Data mining of the AERS database may be a sensitive screening tool, but the reports to MedWatch are of uncertain quality.
How Can a Drug Etiology Be Confirmed?
In addition to clinical criteria (Table 1 ), demonstration of drug-dependent anti-platelet antibodies is important to confirm the etiology of DITP. Because such testing is not widely available and requires substantial time, it is not feasible to wait for test results before deciding whether to discontinue a potential causative drug. Moreover, tests for drug-dependent antibodies can be negative in patients with probable DITP because (1) assay methods may be insufficiently sensitive to detect some antibodies, (2) some drugs are relatively insoluble in water and are difficult to incorporate into in vitro assays, and (3) a metabolite formed in vivo, rather than the primary drug, may be responsible for the thrombocytopenia. However, in most patients in whom a drug etiology is documented by clinical criteria (Table 1 ), drug-dependent platelet-reactive antibodies can be demonstrated. As mentioned, a diagnostic challenge with a suspected drug following recovery can be considered in exceptional circumstances.
What Is the Clinical Course and Appropriate Management of DITP?
DITP typically has an abrupt onset of severe thrombocytopenia. Nadir platelet counts are often less than 20,000/μL, clinically important bleeding is common, and deaths from bleeding have been reported.1;3 The expected course is that recovery of thrombocytopenia begins within 1 to 2 days after the drug is discontinued and recovery is usually complete within a week. Platelet transfusions may be necessary to control overt hemorrhage; corticosteroids are commonly used because the diagnosis of ITP cannot be excluded. When DITP is suspected, it is appropriate to stop corticosteroid therapy abruptly after the platelet count returns to normal. If thrombocytopenia recurs—and it is certain that the patient has not taken the suspected drug again—the diagnosis of DITP is excluded. Drug-dependent antibodies can persist for many years, and patients must be advised to avoid the drug that caused thrombocytopenia indefinitely. For example, acute quinine-induced thrombocytopenia can occur even if the patients have not taken quinine for over 10 years.
What Is the Physician’s Responsibility for Reporting the Association of the Drug with Thrombocytopenia?
When a drug is confirmed as the etiology of thrombocytopenia, it is important for the physician to report this experience (Table 3 ). First, the experience should be reported to the FDA Adverse Event Reporting System by using www.fda.gov/medwatch/. Second, if there are no or few published reports describing definite or probable evidence for the drug as a cause of thrombocytopenia, a case report should be published. These documentations are essential to provide a basis for evaluation of future patients with suspected DITP.
Summary
DITP can be obvious. The management may simply involve stopping the drug (or beverage, food, or herbal remedy) and avoiding its future use. However, much more often the drug etiology for sudden and severe thrombocytopenia is not recognized and inappropriate management is instituted. Awareness of the possibility of DITP is essential in patients presenting with unexpected thrombocytopenia.
Evaluation of published reports on drug-induced thrombocytopenia.
| Adapted from www.ouhsc.edu/platelets. |
| *Criteria for non-evaluable data: insufficient patient data in the report; platelet count not <100,000/μL; cytotoxic drug, marrow suppression; non-therapeutic agent or used in non-therapeutic manner; drug-induced disease in addition to thrombocytopenia; patient age <16 years. |
| Criteria for evaluation of individual patient data |
|
Levels of evidence
|
| Criteria for evaluation of group data |
Levels of evidence
|
| Adapted from www.ouhsc.edu/platelets. |
| *Criteria for non-evaluable data: insufficient patient data in the report; platelet count not <100,000/μL; cytotoxic drug, marrow suppression; non-therapeutic agent or used in non-therapeutic manner; drug-induced disease in addition to thrombocytopenia; patient age <16 years. |
| Criteria for evaluation of individual patient data |
|
Levels of evidence
|
| Criteria for evaluation of group data |
Levels of evidence
|
Drugs that may commonly cause thrombocytopenia.*
| . | Number of reports . | |
|---|---|---|
| Drug (brand name) . | Definite evidence . | Probable evidence . |
| *Data from www.ouhsc.edu/platelets. Drugs were selected for this table because they had 5 or more published reports of individual patient data or group data with definite or probable evidence for a causal relation to thrombocytopenia. | ||
| Abciximab (ReoPro) | 6 | 7 |
| Acetaminophen (Tylenol, Panadol, and others) | 3 | 4 |
| Carbamezapine (Tegretol) | 0 | 10 |
| Chlorpropamide (Diabinese) | 0 | 5 |
| Cimetidine (Tagamet) | 1 | 5 |
| Danazol (Danocrine) | 3 | 4 |
| Diclofenac (Cataflam and Voltaren) | 2 | 3 |
| Efalizumab (Raptiva) | 0 | 6 |
| Eptifibatide (Integrilin) | 2 | 7 |
| Gold (Ridaura, Solganal, and others) | 0 | 11 |
| Hydrochlorothiazide (Aquazide-H, Esidrix, and others) | 0 | 5 |
| Interferon-α (Roferon-A and Intron A) | 1 | 6 |
| Methyldopa (Aldomet) | 3 | 3 |
| Nalidixic Acid (NegGram) | 1 | 5 |
| Quinidine (Quinaglute, Cardioquin, and others) | 26 | 32 |
| Quinine (Quinamm, Quindan, and others) | 14 | 10 |
| Ranitidine (Zantac) | 0 | 5 |
| Rifampin (Rifadin, Rimactane) | 5 | 5 |
| Tirofiban (Aggrestat) | 2 | 6 |
| Trimethoprim/sulfamethoxazole (Bactrim, Septra, and others) | 3 | 12 |
| Vancomycin (Vancoled) | 3 | 4 |
| . | Number of reports . | |
|---|---|---|
| Drug (brand name) . | Definite evidence . | Probable evidence . |
| *Data from www.ouhsc.edu/platelets. Drugs were selected for this table because they had 5 or more published reports of individual patient data or group data with definite or probable evidence for a causal relation to thrombocytopenia. | ||
| Abciximab (ReoPro) | 6 | 7 |
| Acetaminophen (Tylenol, Panadol, and others) | 3 | 4 |
| Carbamezapine (Tegretol) | 0 | 10 |
| Chlorpropamide (Diabinese) | 0 | 5 |
| Cimetidine (Tagamet) | 1 | 5 |
| Danazol (Danocrine) | 3 | 4 |
| Diclofenac (Cataflam and Voltaren) | 2 | 3 |
| Efalizumab (Raptiva) | 0 | 6 |
| Eptifibatide (Integrilin) | 2 | 7 |
| Gold (Ridaura, Solganal, and others) | 0 | 11 |
| Hydrochlorothiazide (Aquazide-H, Esidrix, and others) | 0 | 5 |
| Interferon-α (Roferon-A and Intron A) | 1 | 6 |
| Methyldopa (Aldomet) | 3 | 3 |
| Nalidixic Acid (NegGram) | 1 | 5 |
| Quinidine (Quinaglute, Cardioquin, and others) | 26 | 32 |
| Quinine (Quinamm, Quindan, and others) | 14 | 10 |
| Ranitidine (Zantac) | 0 | 5 |
| Rifampin (Rifadin, Rimactane) | 5 | 5 |
| Tirofiban (Aggrestat) | 2 | 6 |
| Trimethoprim/sulfamethoxazole (Bactrim, Septra, and others) | 3 | 12 |
| Vancomycin (Vancoled) | 3 | 4 |
Evaluation of patients with suspected drug-induced thrombocytopenia.
|
|
A proposed model for drug-dependent antibody binding to an epitope on a platelet glycoprotein. Left: Antibodies capable of causing drug-dependent thrombocytopenia react weakly with an epitope on a glycoprotein. The KA for this interaction is too small to allow significant numbers of antibody molecules to bind in the absence of drug. Right: Drug contains structural elements that are complementary to charged or hydrophobic domains (H) on the glycoprotein epitope and the complementarity determining region (CDR) of the antibody. Drug interacts with the target protein and antibody to improve the “fit” between the two proteins, increasing the KA to a value that permits binding to occur at levels of antibody, antigen and drug achieved in the circulation after ingestion of the drug. Reprinted with permission from Bougie DW et al. Blood. 2006;108:922–927.11)
A proposed model for drug-dependent antibody binding to an epitope on a platelet glycoprotein. Left: Antibodies capable of causing drug-dependent thrombocytopenia react weakly with an epitope on a glycoprotein. The KA for this interaction is too small to allow significant numbers of antibody molecules to bind in the absence of drug. Right: Drug contains structural elements that are complementary to charged or hydrophobic domains (H) on the glycoprotein epitope and the complementarity determining region (CDR) of the antibody. Drug interacts with the target protein and antibody to improve the “fit” between the two proteins, increasing the KA to a value that permits binding to occur at levels of antibody, antigen and drug achieved in the circulation after ingestion of the drug. Reprinted with permission from Bougie DW et al. Blood. 2006;108:922–927.11)
Disclosures Conflict-of-interest disclosures: The authors declare no competing financial interests. label-drug use: None disclosed.
References
Author notes
Department of Medicine, College of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK
Department of Medicine, Medical College of Wisconsin and Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI