Abstract
Essential thrombocythemia (ET) is a hematopoietic disorder that manifests clinically as thrombocytosis, and patients with ET are at increased risk for developing thrombosis, myelofibrosis, and transformation to acute myeloid leukemia. Although ET was recognized as a distinct clinical syndrome more than 6 decades ago and was classified as a myeloproliferative neoplasm (MPN) by William Dameshek in 1951, the molecular pathogenesis of ET remained unknown until 2005, when activating mutations in the JAK2 tyrosine kinase (JAK2V617F) were identified in a significant proportion of patients with ET, polycythemia vera (PV) and primary myelofibrosis (PMF). In addition, subsequent studies have identified gain-of-function mutations in the thrombopoietin receptor (MPL) in a subset of patients with JAK2V617F-negative ET, suggesting that JAK2 activation by distinct mechanisms contributes to the pathogenesis of ET. Despite these important observations, important questions remain regarding the role of JAK2/MPL mutations in ET pathogenesis, the etiology of JAK2/MPL negative ET, the factors that distinguish ET from other MPNs with the JAK2V617F mutation, and the role of JAK2-targeted therapies for the treatment of these MPNs.
Essential Thrombocythemia: Description and Classification
Essential thrombocythemia (ET) is a myeloid disorder that is characterized by an increase in the peripheral blood platelet count that is associated with bone marrow megakaryocyte hyperplasia, without associated erythrocytosis or leukoerythroblastosis.1 ET was first recognized as a distinct clinical syndrome by Emil Epstein and Alfred Godel in 1934,2 and subsequently was recognized as one of the classic myeloproliferative neoplasms (MPN) based on the classification delineated by William Dameshek, the founding editor of Blood, in his seminal editorial in 1951.3 Prior to the identification of JAK2V617F mutations in a significant proportion of patients with polycythemia vera (PV), ET, and primary myelofibrosis (PMF),4–8 a diagnosis of ET required thrombocytosis (platelet count of at least 600 × 109/mL) and megakaryocyte hyperplasia without clinical, pathologic, or molecular evidence supporting a diagnosis of PV, PMF, chronic myeloid leukemia (CML), myelodysplasia (MDS), or reactive thrombocytosis.9 Although the discovery of JAK2V617F is an important advance in our understanding of the molecular pathogenesis of ET (see below), the presence of the JAK2V617F allele in more than one MPN and the absence of the JAK2V617F allele in many patients with ET preclude the use of JAK2V617F testing alone to establish a diagnosis of ET. This is reflected in the revised WHO diagnostic criteria for ET, which lowered the platelet threshold for a diagnosis of ET to 450 × 109/mL, and allows JAK2V617F testing to demonstrate the presence of a clonal MPN, but still requires clinicians to exclude a diagnosis of PV, PMF, CML, or MPN based on clinical, laboratory, and pathologic data.10 It is important to note, however, that it is difficult to consistently apply pathologic criteria to distinguish ET from the other MPN and that it is difficult to distinguish between ET and the prefibrotic/cellular phase of PMF based on clinical, laboratory, or morphologic features.11
JAK2V617F Mutations in ET
Although clonality studies, initially performed through analysis of G6PD isoforms in informative females12 and later through analysis of X-inactivation using PCR-based assays,13,14 demonstrated that PV, ET, and PMF are clonal disorders that originate in multipotent hematopoietic progenitors, the molecular pathogenesis of these MPNs remained an enigma until a series of studies identified the JAK2V617F mutation in PV, ET and PMF. 4–8 These studies demonstrated that the guanine to thymine transversion that leads to a valine to phenylalanine substitution at codon 617 is acquired as a somatic mutation in the hematopoietic compartment. To date, this allele has not been identified in the germline of any patients with MPN, even in kindreds with a familial proclivity for the development of ET and other MPN.15 The absence of germline mutations in JAK2 is, perhaps, not surprising given the importance of JAK2 function to a wide spectrum of cellular functions16 that presumably would be altered by heritable gain-of-function JAK2 mutations.
Subsequent to the identification of the JAK2V617F allele, sensitive, allele-specific assays including BsaXI di-gestion,5 pyrosequencing,17,18 and allele-specific real-time PCR19,20 have been used to assess the frequency of the JAK2V617F allele in ET. Although the exact frequency varies in different studies, the two largest studies to data identified JAK2V617F mutations in 53% to 55% of ET patients.21,22 The majority of studies have analyzed DNA from granulocytes to ascertain the frequency of the JAK2V617F allele, raising the possibility that the hematopoietic compartment affected in ET is not being appropriately assayed. However, two studies have found excellent concordance between granulocyte and platelet JAK2V617F mutational status, and only a handful of patients have been identified in which JAK2V617F is identified in platelet RNA from patients with JAK2V617F-negative granulocytes.21,23 These data suggest that 40% to 50% of ET patients are truly JAK2V617F-negative, validating a search for additional disease alleles in this subset of ET.
Several lines of evidence suggest that there may be differences in quantitative and/or qualitative signaling by JAK2V617F in the different MPNs. First, homozygous JAK2V617F mutations, which result from acquired uniparental disomy of the chromosomal region (9p24) (which includes JAK2),4–7,24 are much more commonly seen in PV than in ET. In addition, when JAK2V617F status is assessed in clonogenic colonies from MPN patients, homozygous JAK2V617F mutant erythroid colonies are present in almost all patients with PV but only rarely in ET.25,26 These data demonstrate that JAK2V617F homozygosity is a common pathogenetic event in PV, but not in ET, and suggest the possibility that the level of JAK2 activity (high in PV, low in ET) determines MPN phenotype. This model is supported by murine studies that show that retroviral overexpression of Jak2V617F in a murine bone marrow transplant (BMT) assay results in polycythemia and leukocytosis without associated thrombocytosis,27–30 whereas lower level JAK2V617F expression in transgenic mouse models results in an ET-like phenotype with thrombocytosis but not polycythemia.31–33 Additional research is needed to determine if cells heterozygous and homozygous for JAK2V617F have differential signaling characteristics that affect MPN phenotype, and to develop more accurate genetic models of Jak2V617F-mediated MPN that will allow for a more detailed assessment of the effects of Jak2V617F gene dosage on phenotype. Although it is likely that gene dosage for JAK2V617F is an important contributor to MPN phenotype, there are data to suggest that additional genetic and epigenetic factors, including germline modifiers34 and epigenetic silencing of JAK-STAT pathway genes,35 contribute to MPN pathogenesis. It is also possible that additional somatic mutations, which remain to be identified, distinguish between JAK2V617F-positive PV, ET, and PMF, and play an important role in instructing the phenotype of JAK2V617F-positive hematopoietic progenitors. The advent of high throughout genomic and epigenomic techniques will facilitate investigation into the pathogenesis of ET, and allow investigators to elucidate the molecular basis for differences in MPN phenotype.
MPLW515 Mutations in ET
Although JAK2 exon 12 mutations are identified in almost all patients with JAK2V617F-negative PV, to date, mutations in JAK2 outside codon 617 have not been identified in JAK2V617F-negative ET and MF,36 suggesting that there are additional, novel, mutations in other genes in this subset of patients. Given the central role of JAK2 in cytokine receptor signaling,16 investigators have asked whether mutations in cytokine receptors can activate JAK2 signaling in JAK2V617F-negative ET and MF. There is precedent for cytokine receptor mutations in hematopoietic disorders, since gain-of-function mutations have been identified in patients with familial erythrocytosis and thrombocytosis.37,38 Sequence analysis of the exons encoding the transmembrane-juxtamambrane domains of the erythropoietin receptor (EPOR), the thrombopoietin receptor (MPL), and the granulocyte-colony stimulating factor receptor (GCSFR) led to the discovery of somatic mutations at codon 515 of MPL in JAK2V617F-negative ET and MF.39–41 Unlike V617F, which is the only substitution to date at codon 617 of JAK2 to be identified in MPN, 3 different mutations at codon 515 have been identified in ET and PMF patients, which result in substitution of leucine, lysine, or alanine for tryptophan.39,40 Recently, Beer and colleagues analyzed the PT-1 study cohort for MPL exon 10 mutations42 and identified somatic MPL mutations in 4.1% of all ET patients, including 8.5% of JAK2V617F-negative ET patients; notably, this included patients with MPLW515L, MPLW515K, and 3 patients with somatic MPLS505N mutations, which had previously been identified as a heritable disease allele in familial thrombocytosis.38 Vannucchi and colleagues screened 994 ET patients for MPLW515L/K mutations and identified MPL mutations in 3% of all ET patients, including 5% of JAK2V617F-negative ET patients.22
MPLW515 lies within the transmembrane-juxta-membrane RWQFP motif, which is thought to maintain the receptor in an inactive conformation in the absence of TPO stimulation, and this conformation is presumably disrupted by these mutations to induce constitutive signaling in the absence of ligand stimulation. In vitro studies are consistent with the notion that MPLW515L is a gain-of-function allele, since expression of MPLW515L transforms hematopoietic cells to cytokine-independent growth, resulting in constitutive activation of JAK2 and other downstream signaling pathways, including STAT3, STAT5, MAPK, and PI3K/AKT,39 as has been similarly observed for JAK2V617F.4 In contrast to the in vitro studies, expression of MPLW515L in vivo in the context of the murine BMT assay causes a phenotype distinct from that induced by JAK2V617F, with marked thrombocytosis and extramedullary hematopoiesis,39 as opposed to the marked polycythemia and normal platelet numbers seen in mice expressing JAK2V617F.27–30 Given that constitutively active MPLW515L signals though wild-type JAK2 and possibly other JAK kinases, it is likely there are differences in signaling between cells transformed by MPLW515 mutations and by JAK2V617F. In addition, it is possible that there are differences in negative feedback on JAK2 signaling, depending on whether the pathogenic mutation is in JAK2 or in the cognate cytokine receptor. Future investigation will likely elucidate the basis for differences in clinical phenotype observed for different alleles in the JAK2 signaling pathway.
In addition, rare patients have been described that have both JAK2V617F and MPLW515L/K mutations.22,40,42 It is unclear whether these mutations are present in the same MPN clone, or if they are present in distinct subclones existing in the same patient. Regardless, the presence of more than one JAK2-activating allele in the same patient raises the intriguing possibilities that 1) heretofore unidentified “pre-JAK2 alleles” predispose to the secondary acquisition of mutations in the JAK2 signaling pathway, 2) that patients with more than one mutation in the JAK2 signaling pathway have an inherited allele that provides a greater selective advantage to cells that acquire mutations in this pathway or 3) that JAK2V617F and MPLW515 mutations can cooperate in MPN pathogenesis. Additional studies are needed to ascertain whether there are inherited or acquired alleles that precede the acquisition of JAK2/MPL alleles in ET patients, and whether there are MPLW515L/ JAK2V617F-double positive clones that contribute to MPN pathogenesis.
Although MPL mutations are only identified in a subset of JAK2V617F-negative ET patients, the identification of mutations upstream of JAK2 in ET/PMF patients demonstrates that activation of JAK-STAT signaling contributes to the pathogenesis of JAK2V617F-positive and JAK2V617F-negative ET. It remains to be seen whether there are additional mutations in the JAK2 pathway in JAK2/MPL-negative MPN; reduced STAT3 phosphorylation has been observed in JAK2V617F-negative ET,43 suggesting that alternate signaling pathways may be involved in the pathogenesis of this subset of ET. The rapid development of JAK2 inhibitors will allow investigators to assess whether MPN patients without known JAK2/MPL mutations are sensitive to JAK2 inhibition in preclinical and clinical studies, and inform genetic studies aimed at elucidating the genetic basis of JAK2/MPL-negative ET. In addition, the development of high throughput genomic platforms, including next generation sequencing, will facilitate future study of the molecular basis of ET and the other MPN.
JAK2/MPL Mutations: Clinical, Molecular, and Therapeutic Correlates
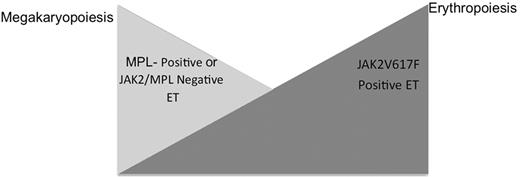
The presence of JAK2V617F and MPL mutations in some, but not all patients with ET has led investigators to determine whether there are clinical and biologic differences that might allow separation of ET patients into clinically distinct, molecularly defined subsets. As discussed above, the largest prospective study of JAK2V617F mutational status in ET was performed by Campbell and colleagues on 776 patients enrolled in the PT-1 trial.21JAK2V617F-positive patients had higher hemoglobin levels, lower platelet counts, and higher neutrophil counts than JAK2V617F-negative patients, as well as lower erythropoietin levels and serum ferritin levels. This led the investigators to suggest that JAK2V617F-positive ET more closely resembles PV, with increased erythropoiesis and granulopoiesis, whereas JAK2V617F-negative ET is characterized by a greater degree of megakaryopoiesis. Of note, these findings have been corroborated in subsequent studies in additional patient cohorts.44 More recently, investigators have assessed the relationship between MPL mutations and clinical and laboratory parameters in ET and found that ET patients with MPL mutations had higher platelet counts and lower hemoglobin levels than patients with JAK2V617F-positive ET, but do not differ from JAK2/MPL wild-type patients in terms of clinical and laboratory parameters.22,42 In addition, several studies have shown that endogenous megakaryocyte, but not erythroid, colonies can be grown from MPLW515-positive patient cells.42,45 These data suggest that ET patients are characterized by variable degrees of megakaryopoiesis and erythropoiesis, with MPLW515-positive or JAK2/MPL-negative disease characterized by a higher degree of megakaryopoiesis and JAK2V617F-positive ET by a higher degree of erythropoiesis (Figure 1 ; see Color Figures, page XX). Moreover, these data suggest there are differences in signal transduction between JAK2V617F and MPLW515L/K that influence clinical phenotype. Although the disease alleles in JAK2/ MPL wild-type ET remain to be identified, patients without known JAK2/MPL mutations are more similar to MPL-positive ET patients than to JAK2V617F-positive ET.
Although many different studies have assessed whether JAK2V617F mutational status and/or quantitative JAK2V617F allele burden influences the risk of thrombosis in ET, to date it has been difficult to ascertain whether JAK2 mutational status has an independent effect on the risk of ET-associated complications. Analysis of the PT-1 cohort suggested that JAK2V617F-positive patients had an increased risk of venous, but not arterial thrombosis.21 More recently, Carobbio and colleagues measured risk factors for thrombosis in a cohort of 657 patients with ET, and found that JAK2V617F allele burden did not confer an increased risk of thrombosis in a multivariate model.46 Instead, they noted that JAK2V617F allele burden correlated with total white blood cell count, and that leukocytosis (white blood cell count greater than 9.4 × 109/L) was predictive of thrombotic risk ; similar findings have recently been observed in PV.47 These data are consistent with recent studies suggesting that leukocyte activation is an important contributor to platelet activation, and suggest that JAK2V617F-mediated effects on thrombosis may, at least in part, be mediated by an increase in neutrophil count and/or increased neutrophil activation.48 A recent study suggested that MPL-positive patients are at increased risk of arterial thrombosis compared to MPL/JAK2-negative ET patients,22 but this was not observed in another study.42 Additional clinical and mechanistic studies are needed to determine whether JAK2V617F and/or MPL mutations contribute to thrombotic risk in ET, directly or indirectly, though effects on neutrophils, platelets, and other factors.
Several recent studies have investigated the relationship between JAK2V617F mutational status and response to therapy. The PT-1 trial randomized high-risk ET patients to receive either hydroxyurea or anagrelide. Although the reduction in platelet counts was similar in both study arms, patients treated with anagrelide were at increased risk of reaching the primary endpoints of the study that included arterial thrombosis, venous thrombosis, hemorrhage, or death from thrombosis.49 In particular, patients treated with anagrelide were at markedly increased risk for arterial thrombosis or hemorrhage, but were at reduced risk for the development of venous thrombosis. Post-hoc JAK2V617F mutational analysis of the PT-1 trial revealed that the increased risk of arterial thrombosis associated with anagrelide therapy was seen in JAK2V617F-positive but not in JAK2V617F-negative ET patients.21 Although these results were obtained retrospectively and have yet to be confirmed in other cohorts, these data are consistent with recent data suggesting that either JAK2V617F-positive disease or a high JAK2V617F allele burden predicts increased sensitivity to hydroxyurea in PV/PMF.50 Thus, the benefits of hydroxyurea over anagrelide may be specific to JAK2V617F-positive ET. Additional studies are needed to confirm this observation, and underlines the importance of incorporating JAK2V617F and MPL testing into clinical trial design of novel agents in ET in order to ascertain whether molecular abnormalities predict response, or lack of response, to specific therapies.
Targeted Therapy for MPNs
The identification of somatic mutations that constitutively activate JAK2 signal transduction provided a rational target for the development of novel therapies for patients with PV, ET, and PMF, and there is considerable optimism that potent, specific inhibitors of JAK2 will be safe and effective in light of the clinical efficacy of ABL kinase inhibitors for the treatment of CML.51–56 Specific inhibitors of JAK2 kinase activity have been designed57–59 and have entered the clinic in Phase I/II trials in PMF and post-PV/ET myelofibrosis.60 Given the central role of JAK2 signaling to a myriad of cellular processes,16 there may be significant toxicities associated with JAK2 inhibition, and “off-target” inhibition of JAK1, JAK3, or TYK2 might lead to hematologic, immunologic, and endocrine side effects. Such toxicities would likely preclude their use in PV and ET, given the indolent nature of these disorders and the relative efficacy and safety of existing therapies. Consequently, it is likely that JAK2 inhibitors will have to demonstrate favorable side effect profiles in PMF and post PV/ET myelofibrosis before being tested in PV and ET.
Phase I trials with JAK2 inhibitors have been initiated in PMF and post-PV/ET myelofibrosis.60,61 The most extensive clinical experience to date is with ICNB018424, a specific JAK2 inhibitor that entered early phase clinical trials in the second half of 2007.60 ICNB018424 is orally bioavailable, and inhibits JAK1 and JAK2, but not JAK3 or TYK2 at clinically achievable concentrations. PMF and post PV/ET MF patients treated with this compound have experienced reductions in splenomegaly and marked improvements in constitutional symptoms, performance status, and body weight. To date, there have only been modest reductions in JAK2V617F allele burden. In addition, patients treated at higher doses of ICNB018424 developed thrombocytopenia that reversed upon dose interruption/ reduction. These results provide important evidence of clinical efficacy of ICNB018424 in patients with PMF and post-PV/ET MF, but many important questions remain regarding the efficacy of this agent, and other JAK2-specific agents. Is the thrombocytopenia observed with ICNB018424 an effect of JAK2 inhibition (presumably through inhibition of TPO signaling), and will modifications in schedule, or use of other JAK2-specific agents, allow for increased target inhibition with less thrombocytopenia? Are the modest effects on JAK2V617F allele burden due to insufficient target inhibition, the relatively short duration of therapy thus far, or due to incomplete dependence on JAK2 signaling by the mutant clone? Are there deleterious effects of chronic JAK1 inhibition? Are the rapid reduction in spleen size and improvement in constitutional symptoms due to effects on the JAK2V617F-mutant clone, or the result of reductions in inflammatory cytokines (which have been shown to be reduced with ICBN018424 therapy)? Although many important questions regarding the role of JAK2-directed therapy for the treatment of PV, ET, and PMF remain, the ongoing clinical development of ICNB018424 and other JAK2-specific inhibitors will provide critical insight into the clinical utility of these agents and into the validity of JAK2 as a therapeutic target in these disorders.
In vitro studies suggest that JAK2 inhibitors will also be of value for patients with JAK2 exon 12 mutations or with MPLW515L/K mutations,62 and we believe that demonstration of clinical activity of JAK2 inhibitors in patients with JAK2/MPL-negative MPN would provide the best evidence that JAK-STAT signaling plays a central and, perhaps, universal role in the pathogenesis of PV, ET, and PMF. Moreover, it is almost certain that some patients will develop resistance to JAK2-inhibitor therapy, possibly through the emergence of resistance mutations, which can be predicted through the use of in vitro studies to predict mechanisms of resistance.63 In addition, the data demonstrating that JAK2V617F-negative AML can emerge in the setting of JAK2V617F-positive MPN clone,64,65 suggests JAK2 inhibitor therapy may not attenuate the risk of leukemic transformation.
Although considerable efforts are being directed towards the preclinical and clinical development of JAK2 inhibitors for the treatment of PV, ET, and PMF, recent data suggests there are additional therapeutic agents that target the MPN clone and may be of value for patients with these disorders. For example, recent preclinical studies suggest that HDAC inhibitors selectively inhibit JAK2V617F-positive cell growth.66 In addition, Kiladjian and colleagues have shown that pegylated interferon alfa-2a therapy leads to complete hematological responses and to marked reductions in JAK2V617F allele burden in PV, with less than 0.1% JAK2V617F allele burden in 7 of 40 patients.67 These data suggest that these agents, alone or in combination with JAK2 inhibitors, may offer significant clinical benefit to patients with ET and with other MPN.
Future Directions
Although the recent discovery of JAK2V617F and MPL mutations have provided important insight into the biology of ET, there are important biologic and clinical questions that remain to be elucidated. The ability of JAK2V617F to contribute to three different MPN, and the specific role of JAK2V617F in ET pathogenesis, remain to be delineated, and the etiology of JAK2/MPL-negative ET requires additional investigation. Most important, the ongoing development of JAK2 inhibitors represents an exciting opportunity to assess whether investigation into the biology of these diseases can realize clinical impact for patients with PV, ET, and PMF.
Model of relative erythropoiesis and megakaryopoiesis based on JAK2/MPL mutational status in essential thrombocythemia (ET). JAK2V617F positive ET is characterized by a relative increase in erythropoiesis, which manifests as a higher hemoglobin level and reduced erythropoietin level, whereas MPL-positive disease or MPL/ JAK2-negative disease is associated with higher platelet counts and reduced hematocrit.
Model of relative erythropoiesis and megakaryopoiesis based on JAK2/MPL mutational status in essential thrombocythemia (ET). JAK2V617F positive ET is characterized by a relative increase in erythropoiesis, which manifests as a higher hemoglobin level and reduced erythropoietin level, whereas MPL-positive disease or MPL/ JAK2-negative disease is associated with higher platelet counts and reduced hematocrit.
Disclosures Conflict-of-interest disclosure: R.L.L. is a consultant for Novartis. M.H. is a consultant for Medco and Bayer; he receives research funding from Novartis. Off-label drug use: None disclosed.
Acknowledgments
We would like to acknowledge the patients and investigators who have contributed to our understanding of these disorders, and apologize to our colleagues whose work was not highlighted due to space constraints. The Levine Laboratory is supported by the US National Institutes of Health, the Howard Hughes Medical Institute Early Career Award Program, and the Doris Duke Charitable Foundation Clinical Scientist Development Award Program. R.L.L. is an American Society of Hematology Basic Research Fellow and is the Geoffrey Beene Junior Chair at Memorial Sloan Kettering Cancer Center.
References
Author notes
Human Oncology and Pathogenesis Program
Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY