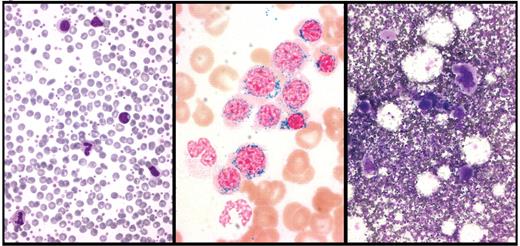
Peripheral blood and bone marrow smears from a patient with refractory anemia with ringed sideroblasts and thrombocytosis (RARS-T). Left panel: peripheral blood smear showing abundant platelets. Middle panel: Perls staining of bone marrow smear showing numerous ringed sideroblasts. Right panel: May Grünwald-Giemsa staining of bone marrow smear showing abnormal megakaryocytes. Courtesy of Rosangela Invernizzi.
Peripheral blood and bone marrow smears from a patient with refractory anemia with ringed sideroblasts and thrombocytosis (RARS-T). Left panel: peripheral blood smear showing abundant platelets. Middle panel: Perls staining of bone marrow smear showing numerous ringed sideroblasts. Right panel: May Grünwald-Giemsa staining of bone marrow smear showing abnormal megakaryocytes. Courtesy of Rosangela Invernizzi.
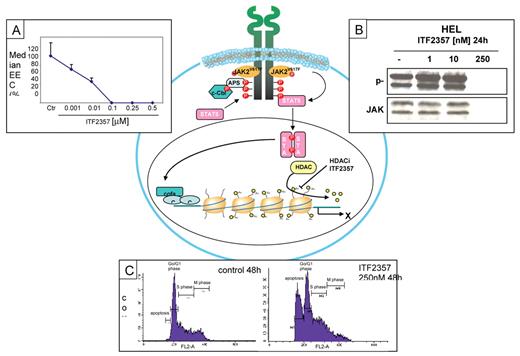
The histone deacetylase (HDAC) inhibitor ITF2357 can inhibit the removal of acetyl groups and the process that leads to chromatin condensation and transcriptional repression. As a consequence, ITF2357 blocks the clonogenic activity of peripheral blood progenitor cells isolated from patients bearing the JAK2V617F mutation (Panel A). Moreover, exposure to ITF2357 (250 nM) is followed by rapid degradation of either total and phosphorylated JAK2 protein in JAK2V617F-mutated HEL cells (Panel B). Finally, at the same concentration, ITF2357 increases the proportion of apoptotic cells and reduces cells in M phase (Panel C).
The histone deacetylase (HDAC) inhibitor ITF2357 can inhibit the removal of acetyl groups and the process that leads to chromatin condensation and transcriptional repression. As a consequence, ITF2357 blocks the clonogenic activity of peripheral blood progenitor cells isolated from patients bearing the JAK2V617F mutation (Panel A). Moreover, exposure to ITF2357 (250 nM) is followed by rapid degradation of either total and phosphorylated JAK2 protein in JAK2V617F-mutated HEL cells (Panel B). Finally, at the same concentration, ITF2357 increases the proportion of apoptotic cells and reduces cells in M phase (Panel C).
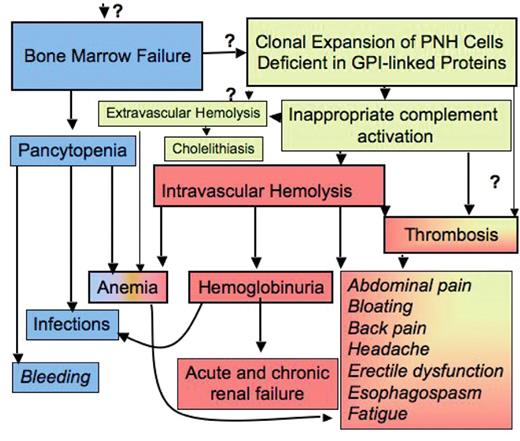
The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria (PNH). Bone marrow failure and the occurrence of PNH blood cells are the two major components in the pathophysiology of PNH and explain the major clinical symptoms of patients with this disease, which are pancytopenia, hemolysis and thrombosis (see also text). The demonstration of blood cells deficient in GPI-linked proteins is sufficient for the diagnosis of the disease. Immune suppressive treatment improves bone marrow failure but does not influence the existence of PNH cells, whereas inhibition of intravascular hemolysis improves hemolysis-associated symptoms, but has no effect on bone marrow failure or on extravascular hemolysis. The increased understanding of the pathophysiology has greatly improved diagnosis and treatment of patients with this disease. However, a few critical “?” remain that need to be solved in order to fully cure the disease.
The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria (PNH). Bone marrow failure and the occurrence of PNH blood cells are the two major components in the pathophysiology of PNH and explain the major clinical symptoms of patients with this disease, which are pancytopenia, hemolysis and thrombosis (see also text). The demonstration of blood cells deficient in GPI-linked proteins is sufficient for the diagnosis of the disease. Immune suppressive treatment improves bone marrow failure but does not influence the existence of PNH cells, whereas inhibition of intravascular hemolysis improves hemolysis-associated symptoms, but has no effect on bone marrow failure or on extravascular hemolysis. The increased understanding of the pathophysiology has greatly improved diagnosis and treatment of patients with this disease. However, a few critical “?” remain that need to be solved in order to fully cure the disease.
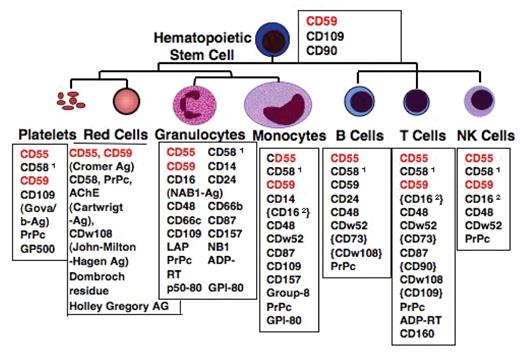
GPI-anchored surface proteins on human hematopoietic cells. Blood group antigens are shown in brackets “{}.” Expression only upon activation or only in a subset of the cells is displayed in parentheses “().” Abbreviations: PrPc, prion protein; AChE, acetylcholinesterase; LAP, leukocyte alkaline phosphatase; EDN, eosinophil-derived neurotoxin; ADP-RT, mono ADP-ribosyl transferase 1both in a GPI-linked and a transmembrane form 2transmembrane-anchored isoform.
GPI-anchored surface proteins on human hematopoietic cells. Blood group antigens are shown in brackets “{}.” Expression only upon activation or only in a subset of the cells is displayed in parentheses “().” Abbreviations: PrPc, prion protein; AChE, acetylcholinesterase; LAP, leukocyte alkaline phosphatase; EDN, eosinophil-derived neurotoxin; ADP-RT, mono ADP-ribosyl transferase 1both in a GPI-linked and a transmembrane form 2transmembrane-anchored isoform.
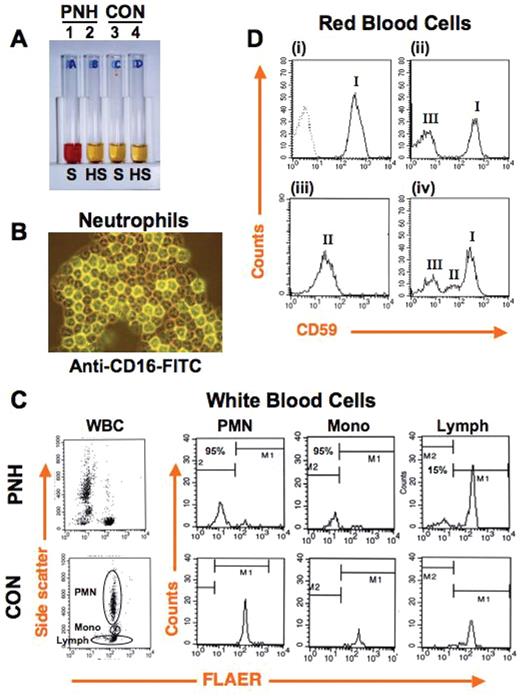
Diagnosis of paroxysmal nocturnal hemoglobinuria (PNH). (A) Ham test, the traditional clinical assay for diagnosis of PNH, is based on the increased susceptibility of PNH red blood cells to complement-mediated lysis. PNH (tubes 1, 2) and control (tubes 3, 4) red blood cells are incubated with serum (S), or serum in which complement has been heat inactivated (HS). Cells and debris are pelleted, and the degree of lysis is assessed by the presence of red pigment from hemoglobin in the serum. Only PNH red blood cells show complement-dependent lysis (tube 1), due to their lack of expression of GPI-linked complement inhibitors CD55 and CD59, while control red blood cells are protected (tube 3). (B) Granulocytes from a PNH patient were stained with fluorescently labeled antibody against the GPI-linked protein CD16. Strongly positive (normal) and completely negative (PNH) cells are present. Typically, populations of both normal and PNH hematopoietic cells reside simultaneously in the peripheral blood. (C) Diagnosis of PNH by using flow cytometry. All hematopoietic lineages can be affected by PNH. Currently, clinical assays often assess PNH based on staining of white blood cells with fluorescently labeled antibodies against at least two GPI-linked proteins or, as shown here using FLAER, a fluorescently labeled derivative of the bacterial protein aerolysin, which naturally binds the GPI anchor. On the left the dot plot analysis of total peripheral blood white blood cells (WBC) from PNH (top) and control (bottom) individuals are shown. Note that in the sample from the PNH patient a proportion of cells have a decreased binding of FLAER (PNH cells). To the right the histograms of specific white blood cell lineages seen in the associated dot plots show substantially reduced FLAER binding to PNH (top) on 95% of neutrophils (PMN) and monocytes (Mono), 15% of lymphocytes (Lymph), as compared to control (bottom). (D) FACS analysis of red blood cells using fluorescently labeled anti-CD59 is used to assess the PNH red cell population and the degree of GPI-anchor deficiency. Normal individuals show uniformly strong staining of red blood cells with CD59 ((i), hatched line shows isotype-matched control antibody). A classic PNH patient (ii) shows distinct populations of type III cells, which are completely deficient in surface expression of GPI-linked proteins, and type I cells, which show normal expression of GPI-linked proteins. Type II cells show a partial deficiency of GPI-linked protein surface expression and can be seen in PNH patients either alone (iii) or in combination with type III and/or type I populations (iv).
Diagnosis of paroxysmal nocturnal hemoglobinuria (PNH). (A) Ham test, the traditional clinical assay for diagnosis of PNH, is based on the increased susceptibility of PNH red blood cells to complement-mediated lysis. PNH (tubes 1, 2) and control (tubes 3, 4) red blood cells are incubated with serum (S), or serum in which complement has been heat inactivated (HS). Cells and debris are pelleted, and the degree of lysis is assessed by the presence of red pigment from hemoglobin in the serum. Only PNH red blood cells show complement-dependent lysis (tube 1), due to their lack of expression of GPI-linked complement inhibitors CD55 and CD59, while control red blood cells are protected (tube 3). (B) Granulocytes from a PNH patient were stained with fluorescently labeled antibody against the GPI-linked protein CD16. Strongly positive (normal) and completely negative (PNH) cells are present. Typically, populations of both normal and PNH hematopoietic cells reside simultaneously in the peripheral blood. (C) Diagnosis of PNH by using flow cytometry. All hematopoietic lineages can be affected by PNH. Currently, clinical assays often assess PNH based on staining of white blood cells with fluorescently labeled antibodies against at least two GPI-linked proteins or, as shown here using FLAER, a fluorescently labeled derivative of the bacterial protein aerolysin, which naturally binds the GPI anchor. On the left the dot plot analysis of total peripheral blood white blood cells (WBC) from PNH (top) and control (bottom) individuals are shown. Note that in the sample from the PNH patient a proportion of cells have a decreased binding of FLAER (PNH cells). To the right the histograms of specific white blood cell lineages seen in the associated dot plots show substantially reduced FLAER binding to PNH (top) on 95% of neutrophils (PMN) and monocytes (Mono), 15% of lymphocytes (Lymph), as compared to control (bottom). (D) FACS analysis of red blood cells using fluorescently labeled anti-CD59 is used to assess the PNH red cell population and the degree of GPI-anchor deficiency. Normal individuals show uniformly strong staining of red blood cells with CD59 ((i), hatched line shows isotype-matched control antibody). A classic PNH patient (ii) shows distinct populations of type III cells, which are completely deficient in surface expression of GPI-linked proteins, and type I cells, which show normal expression of GPI-linked proteins. Type II cells show a partial deficiency of GPI-linked protein surface expression and can be seen in PNH patients either alone (iii) or in combination with type III and/or type I populations (iv).
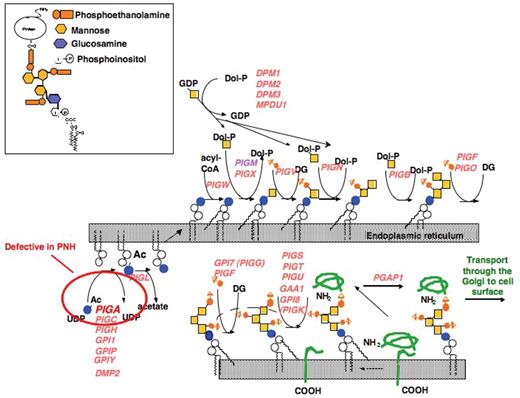
Synthesis of the GPI-precursors proceeds in a stepwise manner. Over 29 proteins have been shown to be involved in the biosynthetic pathway of GPI-anchor biosynthesis.15 It starts on the cytoplasmic side of the endoplasmic reticulum (ER). The addition of the GPI-anchor precursor to the carboxy-terminus of the protein from which a block of 17–31 amino acids has been cleaved occurs on the luminal side of the ER. Insert shows the structure of mammalian GPI-anchored proteins. GPI-anchors have a conserved structure (NH2-CH2-CH2-PO4-6Manα1–2Manα1–6Manα1–4GlcNα1–6myo-inositol-PO4-lipid), which can be modified by the addition of an acyl chain to the inositol (not shown) and phosphoethanolamine to the first and possibly second mannose.
Synthesis of the GPI-precursors proceeds in a stepwise manner. Over 29 proteins have been shown to be involved in the biosynthetic pathway of GPI-anchor biosynthesis.15 It starts on the cytoplasmic side of the endoplasmic reticulum (ER). The addition of the GPI-anchor precursor to the carboxy-terminus of the protein from which a block of 17–31 amino acids has been cleaved occurs on the luminal side of the ER. Insert shows the structure of mammalian GPI-anchored proteins. GPI-anchors have a conserved structure (NH2-CH2-CH2-PO4-6Manα1–2Manα1–6Manα1–4GlcNα1–6myo-inositol-PO4-lipid), which can be modified by the addition of an acyl chain to the inositol (not shown) and phosphoethanolamine to the first and possibly second mannose.
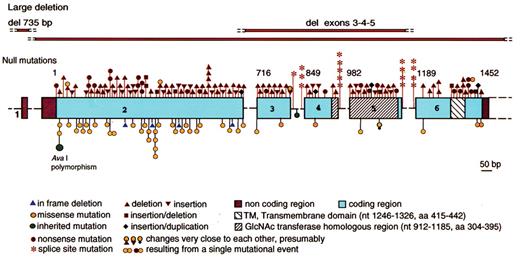
Schematic representation of the structure and mutations in thePIGAgene. Coding region is shown in boxes, introns in form of dashes (not in scale), nucleotide numbers are shown above the exons. Null mutations (frame-shift, nonsense and splicing) are indicated above. Missense mutations and in-frame mutations are shown below. All mutations are somatic mutations with the exception of polymorphism shown in green.
Reproduced with permission from Luzzatto L and Nafa K. Genetics of PNH. In: Young NS, Moss J, eds. PNH and the GPI-linked Proteins. San Diego: Academic Press; 2000:21–47.
Schematic representation of the structure and mutations in thePIGAgene. Coding region is shown in boxes, introns in form of dashes (not in scale), nucleotide numbers are shown above the exons. Null mutations (frame-shift, nonsense and splicing) are indicated above. Missense mutations and in-frame mutations are shown below. All mutations are somatic mutations with the exception of polymorphism shown in green.
Reproduced with permission from Luzzatto L and Nafa K. Genetics of PNH. In: Young NS, Moss J, eds. PNH and the GPI-linked Proteins. San Diego: Academic Press; 2000:21–47.
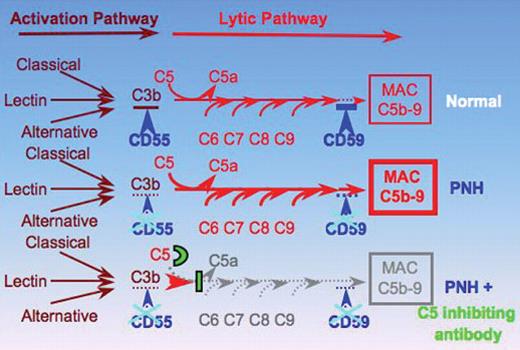
Schematic representation of the complement cascade in normal individuals, in patients with paroxysmal nocturnal hemoglobinuria (PNH), and in patients with PNH receiving the C5 inhibiting antibody eculizumab (modified from Bessler 2005).49 In humans the complement system consists of more than 30 plasma and cell-surface proteins and may be activated by three different pathways: the classical, lectin, and alternative pathway. The classical pathway is activated by antibody–antigen complexes, the lectin pathway involves carbohydrate recognition by pattern-recognition receptors, such as mannose-binding lectin (MBL) and the subsequent activation of MBL-associated serine proteases. The alternative pathway does not involve specific recognition molecules. It is initiated by the covalent binding of a small amount of C3 to hydroxyl or amine groups on the cell-surface molecules of microorganisms. This pathway also functions to amplify the activation of C3. The primary goal of the activation pathway is C3b deposition on the target cell. The activation pathway is followed by the formation of the membrane attack complex (MAC) in the lytic pathway of complement activation, which is necessary for the lysis of the target: C3b, once deposited on the target cells, becomes part of a C5 convertase. After C5b is formed by the C5 convertase, no further proteolytic reaction occurs in the complement cascade. The assembly of the MAC is formed by protein-protein interaction. The deficiency of the surface proteins CD55 and CD59 leads to the uncontrolled lysis of PNH red cells by complement. The C5 binding antibody blocks the lytic pathway of complement activation. Reproduced with permission from Bessler M. Inside blood.
Schematic representation of the complement cascade in normal individuals, in patients with paroxysmal nocturnal hemoglobinuria (PNH), and in patients with PNH receiving the C5 inhibiting antibody eculizumab (modified from Bessler 2005).49 In humans the complement system consists of more than 30 plasma and cell-surface proteins and may be activated by three different pathways: the classical, lectin, and alternative pathway. The classical pathway is activated by antibody–antigen complexes, the lectin pathway involves carbohydrate recognition by pattern-recognition receptors, such as mannose-binding lectin (MBL) and the subsequent activation of MBL-associated serine proteases. The alternative pathway does not involve specific recognition molecules. It is initiated by the covalent binding of a small amount of C3 to hydroxyl or amine groups on the cell-surface molecules of microorganisms. This pathway also functions to amplify the activation of C3. The primary goal of the activation pathway is C3b deposition on the target cell. The activation pathway is followed by the formation of the membrane attack complex (MAC) in the lytic pathway of complement activation, which is necessary for the lysis of the target: C3b, once deposited on the target cells, becomes part of a C5 convertase. After C5b is formed by the C5 convertase, no further proteolytic reaction occurs in the complement cascade. The assembly of the MAC is formed by protein-protein interaction. The deficiency of the surface proteins CD55 and CD59 leads to the uncontrolled lysis of PNH red cells by complement. The C5 binding antibody blocks the lytic pathway of complement activation. Reproduced with permission from Bessler M. Inside blood.
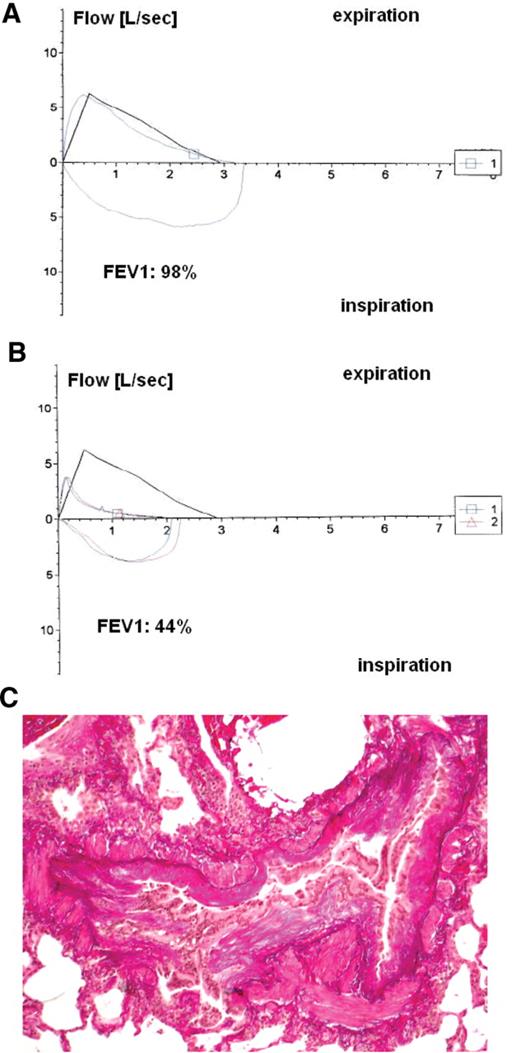
Pulmonary function tests and histology of a patient with bronchiolitis obliterans after hematopoietic stem cell transplantation (HSCT). Flow-volume loop of a forced vital capacity maneuver. Positive values represent expiration, negative values represent inspiration. The trace moves clockwise for expiration followed by inspiration. A: normal pulmonary function at one year after HSCT. B: abnormal pulmonary function one year later, with expiration prolongation, decreased forced expiratory volume at one second (FEV1, 44% of the predicted value). C: histology of bronchiolitis obliterans showing dense concentric peribronchial fibrosis with luminal obliteration, but a relative paucity of inflammatory cells. Note the relative sparing of the alveolar spaces (with the courtesy of Prof. Stephan Dirnhofer, University Hospital, Basel, Switzerland)
Pulmonary function tests and histology of a patient with bronchiolitis obliterans after hematopoietic stem cell transplantation (HSCT). Flow-volume loop of a forced vital capacity maneuver. Positive values represent expiration, negative values represent inspiration. The trace moves clockwise for expiration followed by inspiration. A: normal pulmonary function at one year after HSCT. B: abnormal pulmonary function one year later, with expiration prolongation, decreased forced expiratory volume at one second (FEV1, 44% of the predicted value). C: histology of bronchiolitis obliterans showing dense concentric peribronchial fibrosis with luminal obliteration, but a relative paucity of inflammatory cells. Note the relative sparing of the alveolar spaces (with the courtesy of Prof. Stephan Dirnhofer, University Hospital, Basel, Switzerland)
Renal histology showing cyclosporine toxicity. Vacuolation of the proximal tubular epithelium (*), beaded medial hyalinosis in afferent arteriolae (^), three glomerula with global sclerosis and one glomerulum with compensatory hypertrophy (with the courtesy of Prof. Stephan Dirnhofer, University Hospital, Basel, Switzerland).
Renal histology showing cyclosporine toxicity. Vacuolation of the proximal tubular epithelium (*), beaded medial hyalinosis in afferent arteriolae (^), three glomerula with global sclerosis and one glomerulum with compensatory hypertrophy (with the courtesy of Prof. Stephan Dirnhofer, University Hospital, Basel, Switzerland).
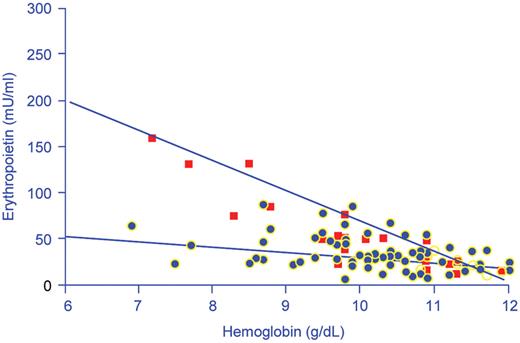
Hemoglobin concentrations in relation to serum immunoreactive erythropoietin concentrations in 74 nonhypoxemic anemic patients with cancer (black squares) and 24 patients with iron deficiency anemia (red squares).
Reproduced with permission from Miller CB, Jones RJ, Piantodosi S et al. N Engl J Med.2
Hemoglobin concentrations in relation to serum immunoreactive erythropoietin concentrations in 74 nonhypoxemic anemic patients with cancer (black squares) and 24 patients with iron deficiency anemia (red squares).
Reproduced with permission from Miller CB, Jones RJ, Piantodosi S et al. N Engl J Med.2
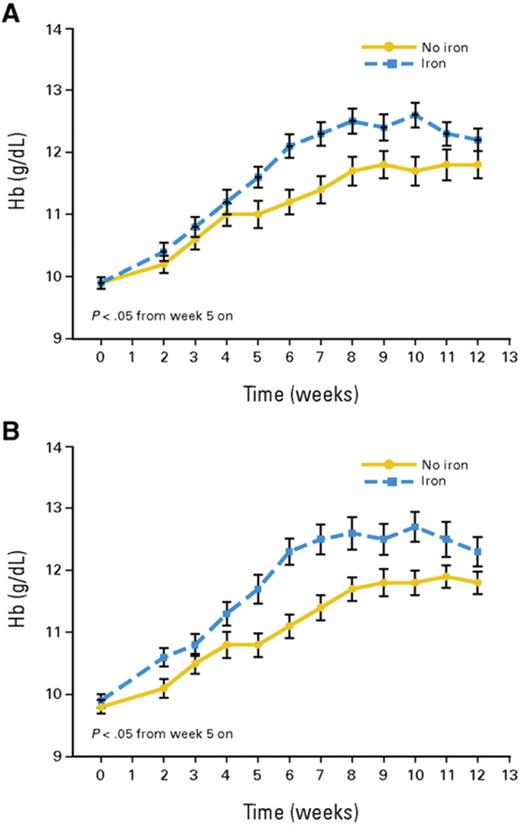
Time course of mean hemoglobin (Hb) in patients receiving ESA only (solid lines) and patients receiving ESA plus IV iron (interrupted lines). A: intention-to-treat population (n = 149); B: per protocol population (n = 103).
Reprinted with permission from
Time course of mean hemoglobin (Hb) in patients receiving ESA only (solid lines) and patients receiving ESA plus IV iron (interrupted lines). A: intention-to-treat population (n = 149); B: per protocol population (n = 103).
Reprinted with permission from
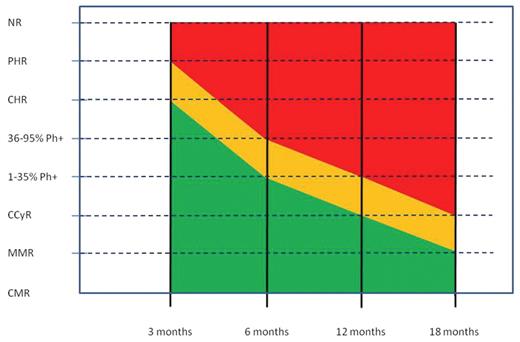
Milestones of therapeutic response in newly diagnosed chronic phase chronic myeloid leukemia (CML) patients treated with standard dose imatinib.
Red shading: failure; orange shading: suboptimal response; green shading: optimal response.
Abbreviations: CCyR,complete cytogenic response; CHR, complete hematologic response; CMR, complete molecular response; MMR, major molecular response; NR, no response; Ph, Philadelphia chromosome; PHR, partial hematologic response.
Milestones of therapeutic response in newly diagnosed chronic phase chronic myeloid leukemia (CML) patients treated with standard dose imatinib.
Red shading: failure; orange shading: suboptimal response; green shading: optimal response.
Abbreviations: CCyR,complete cytogenic response; CHR, complete hematologic response; CMR, complete molecular response; MMR, major molecular response; NR, no response; Ph, Philadelphia chromosome; PHR, partial hematologic response.
Sensitivity of Bcr-Abl kinase domain mutants to Abl kinase inhibitors.
Imatinib: sensitive (≤ 1000 nM), intermediate (≤ 3000 nM), insensitive (> 3000 nM).
Nilotinib: sensitive (≤ 50 nM), intermediate (≤ 500 nM), insensitive (> 500 nM).
Dasatinib: sensitive (≤ 3 nM), intermediate (≤ 60 nM), insensitive (> 60 nM).
* The IC50 value is the concentraion of inhibitor resulting in a 50% reduction in cell viability. For experimental details, see references 8,12,21,29.
† IC50 values from Burgess et al, PNAS 2005.21
‡ IC50 values from Shah et al, Cancer Cell 2002.29
§ IC50 values estimated from Shah et al, Science 2004.8
Sensitivity of Bcr-Abl kinase domain mutants to Abl kinase inhibitors.
Imatinib: sensitive (≤ 1000 nM), intermediate (≤ 3000 nM), insensitive (> 3000 nM).
Nilotinib: sensitive (≤ 50 nM), intermediate (≤ 500 nM), insensitive (> 500 nM).
Dasatinib: sensitive (≤ 3 nM), intermediate (≤ 60 nM), insensitive (> 60 nM).
* The IC50 value is the concentraion of inhibitor resulting in a 50% reduction in cell viability. For experimental details, see references 8,12,21,29.
† IC50 values from Burgess et al, PNAS 2005.21
‡ IC50 values from Shah et al, Cancer Cell 2002.29
§ IC50 values estimated from Shah et al, Science 2004.8
Mechanisms of vasculopathy. Hemolysis, arginine dysregulation, oxidative stress and uncoupled nitric oxide synthase (NOS) are key mechanisms that contribute to the complex vascular pathophysiology of sickle cell disease (SCD). These events limit nitric oxide (NO) bioavailability through several paths that ultimately provoke increased consumption and decreased production of the potent vasodilator, NO. Although often discussed independently, there is significant overlap closely linking these pathways of endothelial dysfunction that prohibit determining cause and effect. The contribution of inflammation coupled with antioxidant, glutathione and Apolipoprotein A-1 (Apo A) depletion, ischemia-reperfusion injury, and acute as well as chronic end-organ damage obscure mechanistic boundaries further. During hemolysis, cell free hemoglobin and arginase are simultaneously released from the erythrocyte and profoundly contribute to low NO bioavailability. Lactate dehydrogenase (LDH) is also released from the erythrocyte and represents a convenient biomarker of hemolysis that delineates the subphenotypes of SCD.
Mechanisms of vasculopathy. Hemolysis, arginine dysregulation, oxidative stress and uncoupled nitric oxide synthase (NOS) are key mechanisms that contribute to the complex vascular pathophysiology of sickle cell disease (SCD). These events limit nitric oxide (NO) bioavailability through several paths that ultimately provoke increased consumption and decreased production of the potent vasodilator, NO. Although often discussed independently, there is significant overlap closely linking these pathways of endothelial dysfunction that prohibit determining cause and effect. The contribution of inflammation coupled with antioxidant, glutathione and Apolipoprotein A-1 (Apo A) depletion, ischemia-reperfusion injury, and acute as well as chronic end-organ damage obscure mechanistic boundaries further. During hemolysis, cell free hemoglobin and arginase are simultaneously released from the erythrocyte and profoundly contribute to low NO bioavailability. Lactate dehydrogenase (LDH) is also released from the erythrocyte and represents a convenient biomarker of hemolysis that delineates the subphenotypes of SCD.
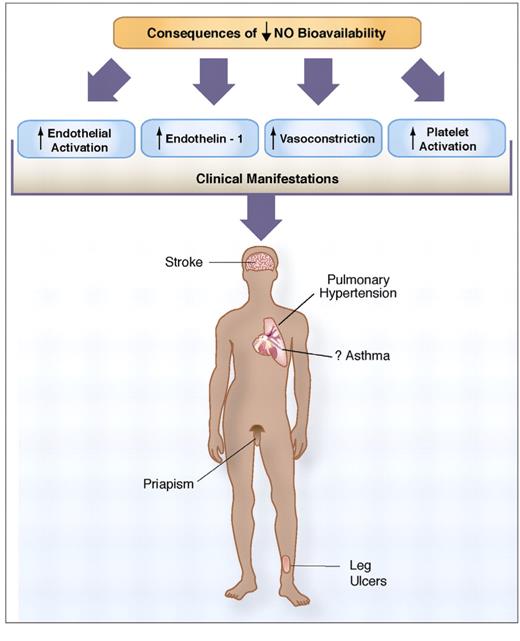
Consequences of low nitric oxide (NO) bioavailability. The consequences of decreased NO bioavailability include endothelial cell activation, upregulation of the potent vasoconstrictor endothelin-1, vasoconstriction, platelet activation, increased tissue factor and activation of coagulation pathways, all of which ultimately translates into the clinical manifestations of sickle cell disease. NO bioavailability is particularly vulnerable to the effects of hemolysis, an event in sickle cell disease that contributes to the development of the hemolytic subphenotypes, which include pulmonary hypertension, priapism, cutaneous leg ulceration, stroke and possibly asthma.
Consequences of low nitric oxide (NO) bioavailability. The consequences of decreased NO bioavailability include endothelial cell activation, upregulation of the potent vasoconstrictor endothelin-1, vasoconstriction, platelet activation, increased tissue factor and activation of coagulation pathways, all of which ultimately translates into the clinical manifestations of sickle cell disease. NO bioavailability is particularly vulnerable to the effects of hemolysis, an event in sickle cell disease that contributes to the development of the hemolytic subphenotypes, which include pulmonary hypertension, priapism, cutaneous leg ulceration, stroke and possibly asthma.
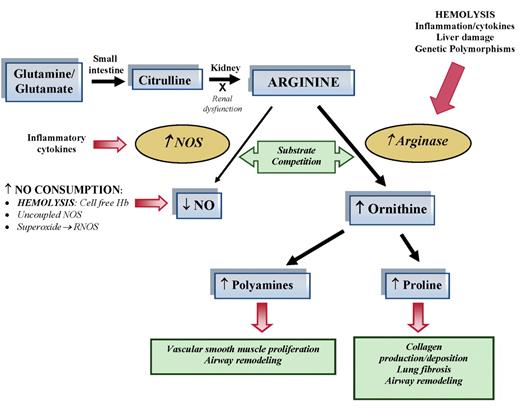
Altered arginine metabolism in hemolysis. Dietary glutamine and glutamate serve as a precursor for the de novo production of arginine through the citrulline-arginine pathway. Arginine is synthesized endogenously from citrulline primarily via the intestinal-renal axis. Arginase and nitric oxide synthase (NOS) compete for arginine, their common substrate. In stem cell disease (SCD) and thalassemia, bioavailability of arginine and nitric oxide (NO) are decreased by several mechanisms linked to hemolysis. The release of erythrocyte arginase during hemolysis increases plasma arginase levels and shifts arginine metabolism towards ornithine production, limiting the amount of substrate available for NO production. The bioavailability of arginine is further diminished by increased ornithine levels because ornithine and arginine compete for the same transporter system for cellular uptake. Despite an increase in NOS, NO bioavailability is low due to low substrate availability, NO scavenging by cell-free hemoglobin released during hemolysis, and through reactions with free radicals such as superoxide and other reactive NO species. Superoxide is elevated in SCD due to low superoxide dismutase activity, high xanthine oxidase activity and potentially as a result of uncoupled NOS in an environment of low arginine and/or tetrahydrobiopterin concentration or insufficient NADPH. Superoxide will react with NO to form reactive NO species (RNOS) including peroxynitrite, which can contribute further to cell damage and cell death. Endothelial dysfunction resulting from NO depletion and increased levels of the downstream products of ornithine metabolism (polyamines and proline) likely contribute to the pathogenesis of lung injury, pulmonary hypertension and asthma in SCD. This model has implications for all hemolytic processes. This new disease paradigm is now recognized as an important mechanism in the pathophysiology of SCD and thalassemia.
Altered arginine metabolism in hemolysis. Dietary glutamine and glutamate serve as a precursor for the de novo production of arginine through the citrulline-arginine pathway. Arginine is synthesized endogenously from citrulline primarily via the intestinal-renal axis. Arginase and nitric oxide synthase (NOS) compete for arginine, their common substrate. In stem cell disease (SCD) and thalassemia, bioavailability of arginine and nitric oxide (NO) are decreased by several mechanisms linked to hemolysis. The release of erythrocyte arginase during hemolysis increases plasma arginase levels and shifts arginine metabolism towards ornithine production, limiting the amount of substrate available for NO production. The bioavailability of arginine is further diminished by increased ornithine levels because ornithine and arginine compete for the same transporter system for cellular uptake. Despite an increase in NOS, NO bioavailability is low due to low substrate availability, NO scavenging by cell-free hemoglobin released during hemolysis, and through reactions with free radicals such as superoxide and other reactive NO species. Superoxide is elevated in SCD due to low superoxide dismutase activity, high xanthine oxidase activity and potentially as a result of uncoupled NOS in an environment of low arginine and/or tetrahydrobiopterin concentration or insufficient NADPH. Superoxide will react with NO to form reactive NO species (RNOS) including peroxynitrite, which can contribute further to cell damage and cell death. Endothelial dysfunction resulting from NO depletion and increased levels of the downstream products of ornithine metabolism (polyamines and proline) likely contribute to the pathogenesis of lung injury, pulmonary hypertension and asthma in SCD. This model has implications for all hemolytic processes. This new disease paradigm is now recognized as an important mechanism in the pathophysiology of SCD and thalassemia.
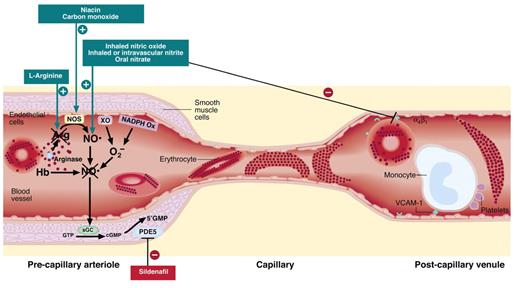
Vascular based therapeutic targets in sickle cell disease (SCD). Several mechanisms have been proposed to contribute to pathological blood flow in SCD: intravascular hemolysis, which releases arginase and hemoglobin (Hb), which respectively reduce levels of the nitric oxide synthase (NOS) substrate arginine and consumes NO; oxygen radicals produced by xanthine oxidoreductase (XO) and NADPH oxidase; sickle hemoglobin polymer formation, inducing stiff, noncompliant red cells; and adhesion of sickle erythrocytes, leukocytes, and platelets to post-capillary venular endothelium. Strategies being tested in clinical trials directed at these pathways are indicated in the boxes and described in detail in the text.
Vascular based therapeutic targets in sickle cell disease (SCD). Several mechanisms have been proposed to contribute to pathological blood flow in SCD: intravascular hemolysis, which releases arginase and hemoglobin (Hb), which respectively reduce levels of the nitric oxide synthase (NOS) substrate arginine and consumes NO; oxygen radicals produced by xanthine oxidoreductase (XO) and NADPH oxidase; sickle hemoglobin polymer formation, inducing stiff, noncompliant red cells; and adhesion of sickle erythrocytes, leukocytes, and platelets to post-capillary venular endothelium. Strategies being tested in clinical trials directed at these pathways are indicated in the boxes and described in detail in the text.
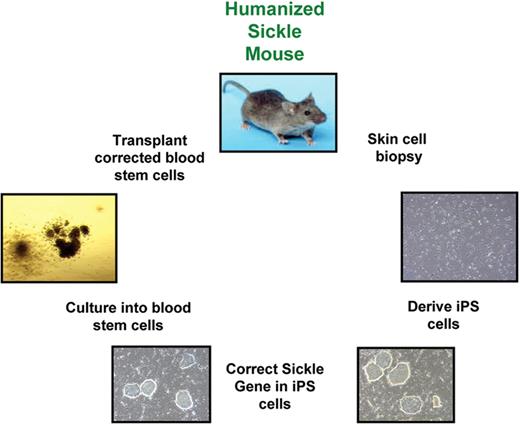
Schematic of gene replacement protocol in iPS cells.
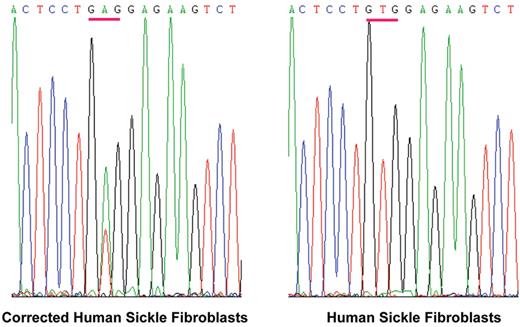
Correction of a βSgene in skin fibroblasts from a sickle cell patient.
Correction of a βSgene in skin fibroblasts from a sickle cell patient.
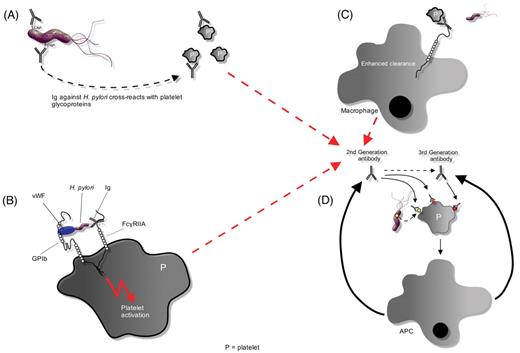
Potential mechanisms ofHelicobacter pylori–induced thrombocytopenia. A) H pylori could induce antibody production in response to antigens, such as CagA, that crossreact against various platelet glycoprotein antigens. B) Platelets may be activated by binding of first-generation H pylori antibodies to platelet FcγRIIA or through an interaction between H pylori–bound von Willebrand factor (VWF) and platelet glycoprotein IB (gpIB). Activation may promote platelet clearance and antigen presentation, which augments production of antibacterial antibodies. C) In the presence of antiplatelet antibodies, the LPS of Gram-negative bacteria can significantly enhance Fc-dependent platelet phagocytosis. D) Epitope spreading and somatic mutation may lead to the development of second- and third-generation antibodies that either recognize bacterially derived factors or crossreact with platelet antigens. The production of these antibodies is no longer dependent upon the stimulation of bacterial antigens, thereby leading to the development of chronic thrombocytopenia refractory to eradication of H pylori infection.
Abbreviations: APC, antigen-presenting cell; P, platelet.
Potential mechanisms ofHelicobacter pylori–induced thrombocytopenia. A) H pylori could induce antibody production in response to antigens, such as CagA, that crossreact against various platelet glycoprotein antigens. B) Platelets may be activated by binding of first-generation H pylori antibodies to platelet FcγRIIA or through an interaction between H pylori–bound von Willebrand factor (VWF) and platelet glycoprotein IB (gpIB). Activation may promote platelet clearance and antigen presentation, which augments production of antibacterial antibodies. C) In the presence of antiplatelet antibodies, the LPS of Gram-negative bacteria can significantly enhance Fc-dependent platelet phagocytosis. D) Epitope spreading and somatic mutation may lead to the development of second- and third-generation antibodies that either recognize bacterially derived factors or crossreact with platelet antigens. The production of these antibodies is no longer dependent upon the stimulation of bacterial antigens, thereby leading to the development of chronic thrombocytopenia refractory to eradication of H pylori infection.
Abbreviations: APC, antigen-presenting cell; P, platelet.
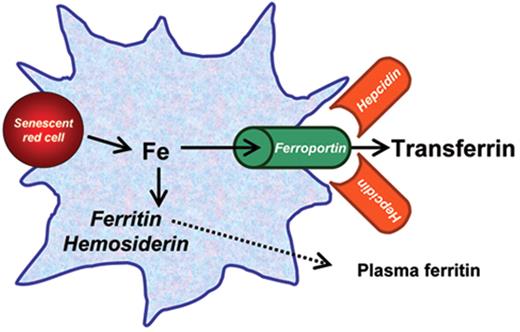
Reticuloendothelial iron metabolism and recycling. Reticuloendothelial cells (macrophages in the bone marrow and spleen, Kupffer cells in the liver) receive their iron from the phagocytosis of nonviable or senescent red blood cells. Heme is catabolized by heme oxygenase 1, and the processed iron is either rapidly recycled to the plasma through the iron exporter ferroportin, or is stored in cytoplasmic ferritin or hemosiderin. This latter is an aggregated and partially denatured form of cytoplasmic ferritin enclosed in membrane-encapsulated lysosomes (siderosomes). Plasma ferritin is a small fraction of cytoplasmic ferritin, glycosylated and secreted through the Golgi apparatus: its concentration reflects the amount of ferritin (and iron) in the macrophage. Ferroportin is the iron exporter from the cell to plasma transferrin: hepcidin, a small peptide produced in the liver, regulates cellular iron efflux by binding to and inducing the degradation via internalization of ferroportin.
In anemic patients receiving regular blood transfusions, iron is primarily taken up by the reticuloendothelial cells and then exported by ferroportin to plasma transferrin, and thereby redistributed to parenchymal cells. This redistribution is modulated by several factors, including the degree of ineffective erythropoiesis through its suppressive effect on hepcidin production.
Reticuloendothelial iron metabolism and recycling. Reticuloendothelial cells (macrophages in the bone marrow and spleen, Kupffer cells in the liver) receive their iron from the phagocytosis of nonviable or senescent red blood cells. Heme is catabolized by heme oxygenase 1, and the processed iron is either rapidly recycled to the plasma through the iron exporter ferroportin, or is stored in cytoplasmic ferritin or hemosiderin. This latter is an aggregated and partially denatured form of cytoplasmic ferritin enclosed in membrane-encapsulated lysosomes (siderosomes). Plasma ferritin is a small fraction of cytoplasmic ferritin, glycosylated and secreted through the Golgi apparatus: its concentration reflects the amount of ferritin (and iron) in the macrophage. Ferroportin is the iron exporter from the cell to plasma transferrin: hepcidin, a small peptide produced in the liver, regulates cellular iron efflux by binding to and inducing the degradation via internalization of ferroportin.
In anemic patients receiving regular blood transfusions, iron is primarily taken up by the reticuloendothelial cells and then exported by ferroportin to plasma transferrin, and thereby redistributed to parenchymal cells. This redistribution is modulated by several factors, including the degree of ineffective erythropoiesis through its suppressive effect on hepcidin production.
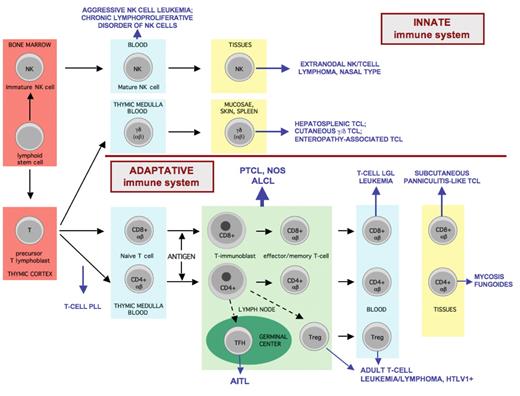
Overview of T-cell and NK-cell differentiation and the putative histogenetic derivation of the major subtypes of mature T- and NK-cell neoplasms.
Mature T cells derived from precursor lymphoblasts in the thymus are genetically characterized by functional rearrangement of the T-cell receptor (TCR) genes. Based on the structure of the TCR, two classes of T cells are recognized: αβ and γδ; both express CD3. γδ T cells are CD4–CD8– or less often CD4–CD8+, they comprise < 5% of T cells in the peripheral blood and show a restricted distribution mainly to the epithelia where they are involved in mucosal immunity, and to the red pulp of the spleen. αβ T cells are divided into CD4+ (mainly helper, with Th1 or Th2 profile of cytokine-secretion) and CD8+ (mainly cytotoxic) subsets. Mature naive T cells are found in the thymic medulla, in the circulation and in the paracortex of lymph nodes. Antigen-dependent reaction occurs in the paracortex of lymph nodes. From the immunoblastic reaction come antigen-specific T cells of either CD4 or CD8 type, as well as memory cells, that may recirculate and home to peripheral tissues.
NK cells derived from a bone marrow precursor are distinguished by the absence of TCR rearrangement and membrane TCR expression. NK cells share some markers with T cells as they can express CD2, CD7, CD43, CD45RO, and cytoplasmic (but not surface) CD3. NK cells are usually CD4–CD8– but may be CD8+, and they express one or several of the “NK-associated” antigens (CD11b, CD16, CD56, CD57, NK receptors), none of which, except perhaps CD16, is specific since they can also be expressed by some T cells. Both NK cells and activated cytotoxic T cells express cytotoxic proteins, T-cell intracellular antigen (TIA)-1, perforin, and granzymeB.
Functionally, the majority of T cells are part of the specific/adaptive immune system and recognize the antigen in a MHC-restricted fashion in the presence of an antigen-presenting cell, whereas NK cells, a subset of the γδ T cells and a minor subset of αβ T cells are part of the innate immunity, i.e., the recognition of antigens occurs through surface receptors independently of a MHC-restricted presentation, involving NK receptors and toll-like receptors.
Mature T/NK-cell lymphomas manifest the immunophenotypic and genetic features of post-thymic T lymphocytes or mature NK cells. T-PLL is thought to derive from naive T cells at a stage intermediate between thymic lymphoblasts and mature naive T cells. NK cells are the cell of origin for aggressive or chronic NK cell leukemias, and give rise to NK-cell lymphomas in tissues, usually in the nasal area. The majority of extranodal T-cell lymphomas derive from cytotoxic T cells; one exception is mycosis fungoides derived from effector CD4+ cells with cutaneous homing properties. γδ T cells give rise to most cases of hepatosplenic T-cell lymphomas as well as a subset of cutaneous T-cell lymphomas. Enteropathy-associated T-cell lymphoma derives from intraepithelial—usually αβ—T cells of the intestine. Adult T-cell lymphoma/leukemia associated with HTLV1 infection is a neoplasm derived from a peculiar subset of CD4+ T cells with a frequent regulatory phenotype (CD25+ FoxP3+). The cellular derivation of nodal-based T-cell lymphomas will be discussed later in the text.
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; LGL, large granular lymphocytic; HTLV, human T-cell leukemia virus; PLL, prolymphocytic leukemia; PTCL, NOS: peripheral T-cell lymphoma, not otherwise specified; TCL, T-cell lymphoma.
Overview of T-cell and NK-cell differentiation and the putative histogenetic derivation of the major subtypes of mature T- and NK-cell neoplasms.
Mature T cells derived from precursor lymphoblasts in the thymus are genetically characterized by functional rearrangement of the T-cell receptor (TCR) genes. Based on the structure of the TCR, two classes of T cells are recognized: αβ and γδ; both express CD3. γδ T cells are CD4–CD8– or less often CD4–CD8+, they comprise < 5% of T cells in the peripheral blood and show a restricted distribution mainly to the epithelia where they are involved in mucosal immunity, and to the red pulp of the spleen. αβ T cells are divided into CD4+ (mainly helper, with Th1 or Th2 profile of cytokine-secretion) and CD8+ (mainly cytotoxic) subsets. Mature naive T cells are found in the thymic medulla, in the circulation and in the paracortex of lymph nodes. Antigen-dependent reaction occurs in the paracortex of lymph nodes. From the immunoblastic reaction come antigen-specific T cells of either CD4 or CD8 type, as well as memory cells, that may recirculate and home to peripheral tissues.
NK cells derived from a bone marrow precursor are distinguished by the absence of TCR rearrangement and membrane TCR expression. NK cells share some markers with T cells as they can express CD2, CD7, CD43, CD45RO, and cytoplasmic (but not surface) CD3. NK cells are usually CD4–CD8– but may be CD8+, and they express one or several of the “NK-associated” antigens (CD11b, CD16, CD56, CD57, NK receptors), none of which, except perhaps CD16, is specific since they can also be expressed by some T cells. Both NK cells and activated cytotoxic T cells express cytotoxic proteins, T-cell intracellular antigen (TIA)-1, perforin, and granzymeB.
Functionally, the majority of T cells are part of the specific/adaptive immune system and recognize the antigen in a MHC-restricted fashion in the presence of an antigen-presenting cell, whereas NK cells, a subset of the γδ T cells and a minor subset of αβ T cells are part of the innate immunity, i.e., the recognition of antigens occurs through surface receptors independently of a MHC-restricted presentation, involving NK receptors and toll-like receptors.
Mature T/NK-cell lymphomas manifest the immunophenotypic and genetic features of post-thymic T lymphocytes or mature NK cells. T-PLL is thought to derive from naive T cells at a stage intermediate between thymic lymphoblasts and mature naive T cells. NK cells are the cell of origin for aggressive or chronic NK cell leukemias, and give rise to NK-cell lymphomas in tissues, usually in the nasal area. The majority of extranodal T-cell lymphomas derive from cytotoxic T cells; one exception is mycosis fungoides derived from effector CD4+ cells with cutaneous homing properties. γδ T cells give rise to most cases of hepatosplenic T-cell lymphomas as well as a subset of cutaneous T-cell lymphomas. Enteropathy-associated T-cell lymphoma derives from intraepithelial—usually αβ—T cells of the intestine. Adult T-cell lymphoma/leukemia associated with HTLV1 infection is a neoplasm derived from a peculiar subset of CD4+ T cells with a frequent regulatory phenotype (CD25+ FoxP3+). The cellular derivation of nodal-based T-cell lymphomas will be discussed later in the text.
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; LGL, large granular lymphocytic; HTLV, human T-cell leukemia virus; PLL, prolymphocytic leukemia; PTCL, NOS: peripheral T-cell lymphoma, not otherwise specified; TCL, T-cell lymphoma.
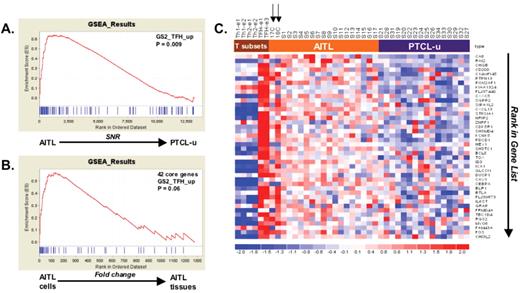
Gene expression profiling demonstrates a molecular link between (angioimmunoblastic T-cell lymphoma) AITL and follicular helper T (TFH) cells (adapted from de Leval et al6).
In this molecular profiling study of 18 AITL and 16 peripheral T-cell lymphomas, not otherwise specified (PTCL, NOS) samples, gene set enrichment analyses (GSEA) were performed by using sets of genes representative of distinct normal T-cell subsets, in order to compare the molecular signature of the tumor samples. Panels A and B show enrichment score (ES) curves generated by such analyses. In panel A, genes discriminant between AITL and PTCL, NOS were ranked (according to the signal to noise ratio) from left to right, and GSEA was performed by using a set of 100 genes overexpressed in normal TFH cells; the ES curve is significant, meaning that the AITL samples display significant enrichment in the TFH signature, as compared to PTCL, NOS. In panel B, two AITL samples consisting of purified tumor cell suspensions were compared with 16 AITL tissue samples, and GSEA showed a significant enrichment of the tumor cell suspensions in the set of the 42 core genes (accounting for the enrichment in panel A). Finally the finding that the imprint of the TFH signature was stronger in purified AITL tumor cells samples compared with AITL tissues, validates that the molecular link with TFH cells was related to the neoplastic cells. Panel C show the associated heatmap of these 42 core genes. Expression data from normal T-cell subsets, 2 AITL tumor cell suspension samples (arrows), 16 AITL tissue samples and 16 PTCL, NOS samples are represented. Standardized expression ranges from −2.0 (blue) to 2.0 (red).
Gene expression profiling demonstrates a molecular link between (angioimmunoblastic T-cell lymphoma) AITL and follicular helper T (TFH) cells (adapted from de Leval et al6).
In this molecular profiling study of 18 AITL and 16 peripheral T-cell lymphomas, not otherwise specified (PTCL, NOS) samples, gene set enrichment analyses (GSEA) were performed by using sets of genes representative of distinct normal T-cell subsets, in order to compare the molecular signature of the tumor samples. Panels A and B show enrichment score (ES) curves generated by such analyses. In panel A, genes discriminant between AITL and PTCL, NOS were ranked (according to the signal to noise ratio) from left to right, and GSEA was performed by using a set of 100 genes overexpressed in normal TFH cells; the ES curve is significant, meaning that the AITL samples display significant enrichment in the TFH signature, as compared to PTCL, NOS. In panel B, two AITL samples consisting of purified tumor cell suspensions were compared with 16 AITL tissue samples, and GSEA showed a significant enrichment of the tumor cell suspensions in the set of the 42 core genes (accounting for the enrichment in panel A). Finally the finding that the imprint of the TFH signature was stronger in purified AITL tumor cells samples compared with AITL tissues, validates that the molecular link with TFH cells was related to the neoplastic cells. Panel C show the associated heatmap of these 42 core genes. Expression data from normal T-cell subsets, 2 AITL tumor cell suspension samples (arrows), 16 AITL tissue samples and 16 PTCL, NOS samples are represented. Standardized expression ranges from −2.0 (blue) to 2.0 (red).
The overlapping spectrum of nodal peripheral T-cell lymphomas (PTCL) entities.
Nodal PTCLs comprise four main entities: angioimmunoblastic T-cell lymphoma (AITL), ALK+anaplastic large cell lymphoma (ALCL), ALK– ALCL and PTCL, not otherwise specified (PTCL, NOS). The only well-demarcated entity is ALK+ ALCL. TFH cells have recently been identified as the cell of origin of AITL, whereas the cellular origin of the other entities is not known precisely. There is a lack of specific definition criteria for PTCL, NOS, and thus substantial overlap with AITL, and ALK– ALCL. Gene expression profiling analyses have identified traces of the TFH signature in a subset of PTCL, NOS, suggesting that the spectrum of AITL may be wider than anticipated. A small group of “follicular” PTCLs, different from AITL, might also derive from TFH cells and could be associated with a distinct translocation t(5 ;9) involving ITK and SYK. The overlap between PTCL, NOS and ALK– ALCL is accounted by a subset of PTCLs composed of large CD30+ cells.
The overlapping spectrum of nodal peripheral T-cell lymphomas (PTCL) entities.
Nodal PTCLs comprise four main entities: angioimmunoblastic T-cell lymphoma (AITL), ALK+anaplastic large cell lymphoma (ALCL), ALK– ALCL and PTCL, not otherwise specified (PTCL, NOS). The only well-demarcated entity is ALK+ ALCL. TFH cells have recently been identified as the cell of origin of AITL, whereas the cellular origin of the other entities is not known precisely. There is a lack of specific definition criteria for PTCL, NOS, and thus substantial overlap with AITL, and ALK– ALCL. Gene expression profiling analyses have identified traces of the TFH signature in a subset of PTCL, NOS, suggesting that the spectrum of AITL may be wider than anticipated. A small group of “follicular” PTCLs, different from AITL, might also derive from TFH cells and could be associated with a distinct translocation t(5 ;9) involving ITK and SYK. The overlap between PTCL, NOS and ALK– ALCL is accounted by a subset of PTCLs composed of large CD30+ cells.
A. Low-power H&E-stained section of a lymph node involved with Burkitt lymphoma (BL) from an 82-year-old man. Note the uniform appearance of the cells, the diffuse pattern of growth, and the nuclear debris from apoptotic cells, mostly contained within the cytoplasm of pale-staining tingible-body macrophages. B. Cytology of bone marrow cells, as seen on Wright-Giemsa–stained aspirates and touch preps. Note the high nuclear-to-cytoplasmic ratio, moderately abundant dark blue cytoplasm with occasional vacuoles, and nucleus with finely granular chromatin and multiple nucleoli. C. Identification of MYC translocation by FISH analysis using “MYC break-apart probes. When together, as in a normal chromosome, the two probes are located in close proximity and give a composite yellow color, while the probes are distinct when translocation occurs (Taken with permission from Hecht and Aster39). D. Composite figure from paper by Hummel et al.6 depicting the results of gene array studies, karyotype and FISH analyses. Top, bar graph depicting the karyotypic complexity of each case. The mBL Index section depicts a heat map of the 58 mBL signature genes; blue is low expression, while yellow is high expression. IG-myc translocations are in bright green; non-IG-myc fusions are in dark green, and myc-absent are in red. At bottom, histologic diagnosis: bright green, BL; dark green, atypical BL; red, diffuse large B-cell lymphoma (DLBCL); grey, unclassifiable mature aggressive B-cell lymphoma.
A. Low-power H&E-stained section of a lymph node involved with Burkitt lymphoma (BL) from an 82-year-old man. Note the uniform appearance of the cells, the diffuse pattern of growth, and the nuclear debris from apoptotic cells, mostly contained within the cytoplasm of pale-staining tingible-body macrophages. B. Cytology of bone marrow cells, as seen on Wright-Giemsa–stained aspirates and touch preps. Note the high nuclear-to-cytoplasmic ratio, moderately abundant dark blue cytoplasm with occasional vacuoles, and nucleus with finely granular chromatin and multiple nucleoli. C. Identification of MYC translocation by FISH analysis using “MYC break-apart probes. When together, as in a normal chromosome, the two probes are located in close proximity and give a composite yellow color, while the probes are distinct when translocation occurs (Taken with permission from Hecht and Aster39). D. Composite figure from paper by Hummel et al.6 depicting the results of gene array studies, karyotype and FISH analyses. Top, bar graph depicting the karyotypic complexity of each case. The mBL Index section depicts a heat map of the 58 mBL signature genes; blue is low expression, while yellow is high expression. IG-myc translocations are in bright green; non-IG-myc fusions are in dark green, and myc-absent are in red. At bottom, histologic diagnosis: bright green, BL; dark green, atypical BL; red, diffuse large B-cell lymphoma (DLBCL); grey, unclassifiable mature aggressive B-cell lymphoma.
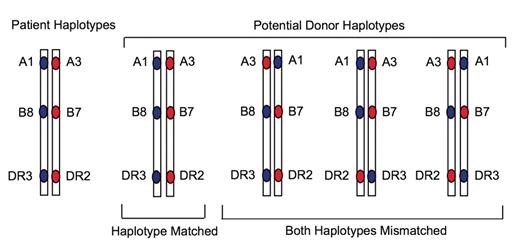
Potential haplotype organization of 6 of 6 matched unrelated donors.14
Potential haplotype organization of 6 of 6 matched unrelated donors.14
Histologic examples of nodal marginal zone lymphoma (NMZL), splenic marginal zone lymphoma (SMZL), and gastric extranodal marginal zone lymphoma (gastric MALT lymphoma). A. Whole mount of a lymph node with NMZL. A thickened capsule (lower left) and interfollicular infiltrate of small B lymphocytes and monocytoid B cells is shown. A reactive follicle (white arrow) is surrounded by an expanded marginal zone of monocytoid B cells while other follicles have been partially replaced by small B cells (black arrows and inset). B. Section of a spleen with SMZL showing marked expansion of the white pulp by monocytoid B cells (inset). Note the lack of follicular mantles and infiltration of monocytoid B cells into the splenic red pulp. C. Full thickness section of gastric MALT lymphoma showing an ulcerated epithelial surface and diffuse infiltrate of marginal zone cells in the lamina propria that have colonized the germinal centers of the B-cell follicles. Scattered lymphoepithelial lesions were also present (inset).
Histologic examples of nodal marginal zone lymphoma (NMZL), splenic marginal zone lymphoma (SMZL), and gastric extranodal marginal zone lymphoma (gastric MALT lymphoma). A. Whole mount of a lymph node with NMZL. A thickened capsule (lower left) and interfollicular infiltrate of small B lymphocytes and monocytoid B cells is shown. A reactive follicle (white arrow) is surrounded by an expanded marginal zone of monocytoid B cells while other follicles have been partially replaced by small B cells (black arrows and inset). B. Section of a spleen with SMZL showing marked expansion of the white pulp by monocytoid B cells (inset). Note the lack of follicular mantles and infiltration of monocytoid B cells into the splenic red pulp. C. Full thickness section of gastric MALT lymphoma showing an ulcerated epithelial surface and diffuse infiltrate of marginal zone cells in the lamina propria that have colonized the germinal centers of the B-cell follicles. Scattered lymphoepithelial lesions were also present (inset).
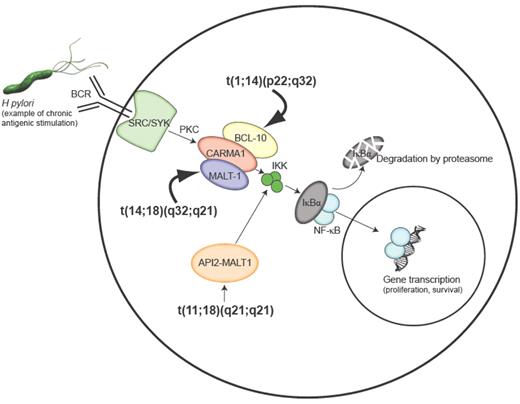
Activation of NF-κB and chromosomal translocations found in MALT lymphoma. Epitopes from Helicobacter pylori can lead to activation of NF-κB through the B-cell receptor (BCR) via receptor-associated tyrosine kinases of the SRC and SYK family, which signal a protein kinase C (PKC) family member to oligomerize BCL-10, CARMA1, and MALT1 leading to activation of the inhibitor of NF-κB kinase (IKK) complex. The IKK complex phosphorylates the inhibitor of NF-κB (IκB) prompting its degradation by the proteasome and allowing the NF-κB family members to translocate to the nucleus and control NF-κB–dependent gene expression. The t(1;14) and t(14;18) translocations lead to overexpression of the BCL10 and MALT1 genes respectively and the t(11;18) translocation produces a chimeric API2-MALT1 protein that can directly activate the IKK complex. Constitutive activation of the NF-κB pathway by these translocations bypasses the requirement for BCR signaling and may account for the antigen independence of cells harboring these translocations.
Activation of NF-κB and chromosomal translocations found in MALT lymphoma. Epitopes from Helicobacter pylori can lead to activation of NF-κB through the B-cell receptor (BCR) via receptor-associated tyrosine kinases of the SRC and SYK family, which signal a protein kinase C (PKC) family member to oligomerize BCL-10, CARMA1, and MALT1 leading to activation of the inhibitor of NF-κB kinase (IKK) complex. The IKK complex phosphorylates the inhibitor of NF-κB (IκB) prompting its degradation by the proteasome and allowing the NF-κB family members to translocate to the nucleus and control NF-κB–dependent gene expression. The t(1;14) and t(14;18) translocations lead to overexpression of the BCL10 and MALT1 genes respectively and the t(11;18) translocation produces a chimeric API2-MALT1 protein that can directly activate the IKK complex. Constitutive activation of the NF-κB pathway by these translocations bypasses the requirement for BCR signaling and may account for the antigen independence of cells harboring these translocations.
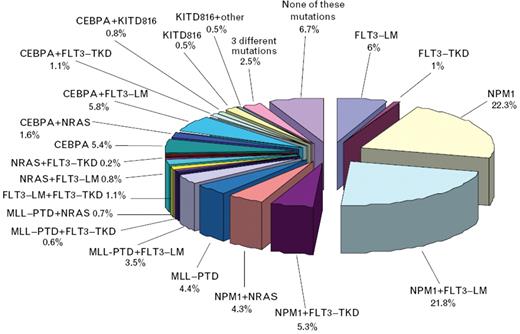
Frequency of molecular markers and their cooperation in patients with acute myeloid leukemia (AML) and normal karyotype (n = 1447).56
Frequency of molecular markers and their cooperation in patients with acute myeloid leukemia (AML) and normal karyotype (n = 1447).56